Class 11 Chemistry Chapter Chemical bonding and molecular structure Notes

It is defined as the attractive forces which hold the various chemical constituents (atoms, ions, etc.) together in different chemical species.
Bond forms to get the stability. with a release of energy.
Kossel-Lewis Approach to Chemical Bonding
According to this theory. atoms take part in the bond formation to complete their octet or to acquire the electronic configuration of the nearest inert gas atoms (Octet rule). This can be achieved by gaining, losing or sharing the electrons.
Lewis Symbols
Valence electrons are reported by dots around the chemical symbol of element, e.g.,
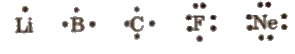
Ionic Bond
A chemical bond formed by complete transference of electrons from one atom (metal) to another (non-metal) and hence, each atom acquire the stable nearest noble gas configuration, is called ionic bond or electrovalent bond, e.g., formation of sodium chloride
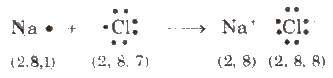
Favourable factors for the formation of ionic bonds
(i) Metal should have lowest ionisation enthalpy.
(ii) Non-metal must have highest electron gain enthalpy.
(iii) The energy released during the formation of 1 mole of crystal lattice, i.e., lattice enthalpy must be high.
[Some elements exhibit variable electrovalency. The reason for this iS unstable configuration of penultimate orbit and inert pair effect].
Ions
Species carrying either positive or negative charge are termed as ions. Species carrying positive charge are called cations and that carrying negative charge are called anions. Metals usually form cation while non-metals (except H) usually form anions.
General Characteristics of Ionic Compounds
1. Ionic compounds are usually solids in nature.
2. Ionic compounds have high melting and boiling points.
3. Ionic compounds are soluble in polar solvents like water but insoluble in non-polar solvents like benzene, C014 etc.
4. Ionic compounds are good conductor in molten state and in aqueous solution.
5. Ionic compounds has crystal structure.
Born Haber Cycle
This cycle is based upon the fact that the formation of an ionic compound may occur either by direct combination of the elements or by an alternate process in which :
1. The reactants (metal) are vaporised to convert into gaseous state.
2. The gaseous atoms are converted into ion.
3. The gaseous ions are combined to form ionic lattice of molecule. e.g., formation of NaCI can be shown as
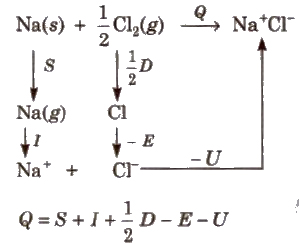
where, S = enthalpy of sublimation
I = ionisation energy
D = dissociation energy
E = electron affinity
U = lattice energy
Q = total enthalpy change
Method of Writing Formula of Ionic Compound
1. Write the symbol of cation at the left and anion at the right.
2. Write their electrovalencies in figures on the top of each symbol as AXBY.
3. Divide their valencies by HCF.
4. Now apply criss-cross rule as  i.e., formula is AyBx.
i.e., formula is AyBx.
 i.e., formula is AyBx.
i.e., formula is AyBx.
e.g., formula of aluminum sulphate  is Al2(So4)3.
is Al2(So4)3.
 is Al2(So4)3.
is Al2(So4)3.
Covalent Bond
A chemical bond formed between two atoms by mutual sharing of electrons between them so as to complete their octets or duplets, is known as covalent bond and the number of electrons contributed by each atom is known as covalency. e.g., formation of CI2/
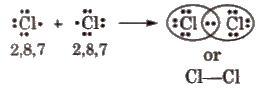
In covalent bonding, the shared pairs of electrons present between the atoms are called bond pairs while unshared pairs or non-bonding electron pairs are known as lone pairs.
Polar Covalent Bond
If a covalent bond is formed between the different ~toms, the shared pair is displaced towards the more electronegative atom causing greater concentration of electron density around the more electronegative atom. Such a covalent bond develops some ionic character and is called polar covalent bond (e.g., H-CI).
Properties of Covalent Compounds
1. In general, covalent compounds exist in the liquid or gaseous state at room temperature due to magnitude of intermolecular forces.
2. Covalent compounds have low melting and boiling points.
3. Covalent compounds are generally poor conductors of electricity because they do not contain free electrons or ions to conduct electricity.
4. They are soluble in non-polar solvents like benzene but usually insoluble in water.
Octet Rule
According to Octet rule during the formation of a covalent bond, the atom attain an inert gas electronic configuration (valence shell contains 8e– or shell is completely filled). An atom may attain this configuration by gaining, losing or sharing electrons with other atoms
Exceptions of the Octet Rule
(i) Incomplete octet of the central atom, e.g., LiCl, BeH2 and BCl3
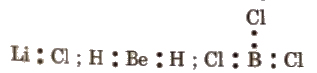
(ii) Odd electron molecules
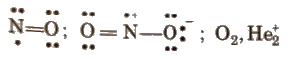
(iii) Expanded octet of central atoms

Formal Charge on an Atom in a Molecule/Ion
Formal charge (F.C) on an atom in a Lewis structure
= [total number of valence electrons in the free atom]
– [total number of Don-bonding (lone pair) electrons]
– 1 / 2 [total number of bonding (shared) electrons].
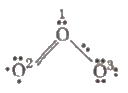
F.C. on O1 = 6 – 2 – 1 / 2 (6) = + 1
Hence, O3 along with the formal charges can be represented as follows
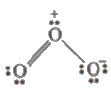
Bond Characteristics
Bond Length
In a covalently bonded molecule. distance between the nuclei of the two atoms is known as bond length. Bond length increases with increases is the size of bonded atoms and decreases with an increase in the number of bonds between bonded atoms.
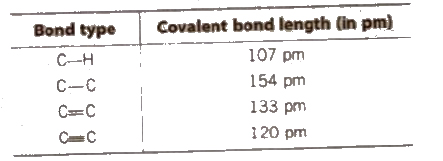
Bond length is determined by X·ray diffraction or electron differences method.
Bond Angle
In a covalently bonded molecule having more than two atoms, the bonds form an angle with each other, which is known as bond angle. In general an increase in the size of central atom decreases the bond angle. Factors affecting bond angle (i) Lone pair repulsion (ii) hybridisation of central room.
It is determined by X-rays diffraction method.
Bond Order
It is defined as the number of covalent bonds present in a molecule.
Bond order = 1 / 2 [Number of electrons in bonding orbitals – Number of electrons in anti-bonding orbitals]
Bond order ∝ 1 / bond length
If bond order comes out to be zero, the molecule does not exist.
Bond Enthalpy
It is the amount of energy released when one mole of covalent bonds is formed while the bond dissociation enthalpy is the amount of energy required to break the one mole of bonds of the same kind so as to separate the bonded atoms in the gaseous state.
The bond enthalpy and bond dissociation enthalpy are equal in magnitude and opposite in sign.
[Bond dissociation enthalpy is determined by thermal or spectroscopic methods.]
As the bond order increases, bond enthalpy also increases and bond length decreases.
Factors affecting bond enthalpy
(i) atomic size
(ii) electronegativity
(iii) extent of overlapping
(iv) bond order
Dipole Moment (μ)
It is defined as the product of the magnitude of the charge and the distance between the centres of positive and negative charge.
μ = charge (Q) x distance of separation (r)
Dipole Moment is expressed in Debye. (D).
1 D = 1 * 10-18 esu-cm = 3.33564 * 10-30 C-m
where, c is coulomb and m is meter.
(The shift in electron density is symbolised by broken arrow)
NH3 has higher dipole moment than NF3.

Resultant dipole moment.
μ = √μ21 + μ22 + 2μ1μ2 cos θ
Applications of Dipole Moment
1. Dipole moment is helpful in predicting the geometry of the molecule.
2. Dipole moment helps in determining the polarity
Hannay-Smith equation
Percent ionic character = 16 [XA – XB] + 3.5 [XA – XB]2
where, XA and XB are the electronegativities of atoms.
Percent ionic character can also be calculated by dipole moment as
Percent ionic character = observed dipole moment / calculated dipole moment * 100
3. Non-polar molecule has zero dipole moment like Bf3, CCI4, etc.
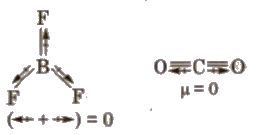
4. cis and trans isomers can be distinguished by dipole moments usually cis isomer have higher dipole moment and hence, higher polarity.
5. Dipole moment is greatest for ortho isomer, zero for para isomer and less than that of ortho for meta isomer.
Fajan’s Rule
The partial covalent character of ionic bonds was discussed by Fajan’s in terms of following rules :
The smaller the size of calion and the larger the size of the anion, the greater the covalent character of an ionic bond,
The greater the charge on the cation or anion, the greater the covalent character of the ionic bond.
Resonance
According to the concept of resonance, a single Lewis structure cannot explain all the properties of the molecules. The molecule is then exposed to have many structures, each of which can explain most of the properties. The actual structure lies in between of all these contributing structures and is called resonance hybrid and the different individual structures are called resonating structures or canonical structures. This phenomenon is known as resonance.
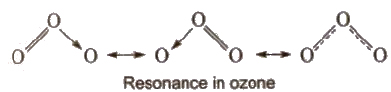
Resonance stabilises the molecule as the energy of the resonance hybrid is less than the energy of any single canonical structure.
Resonance averages the bond characteristics as a whole.
The difference in the energy of the resonance hybrid and the most stable contributing structure (having least energy) is called resonance energy. Greater the resonance energy, greater is the stability of the molecule.
[Calculation of bond order for molecules showing resonance Bond order
= total number of bonds between two atoms in all the structures / total number of resonating structures]
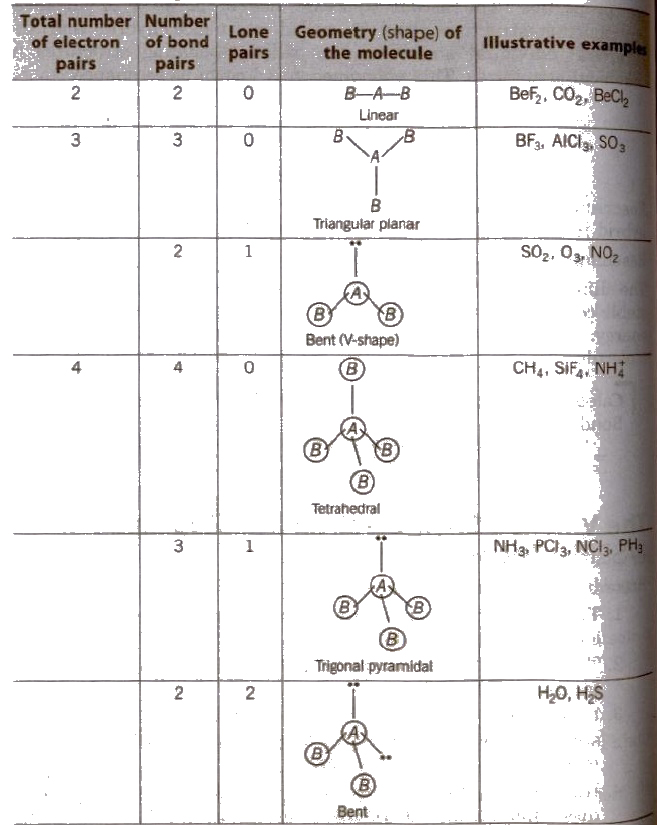
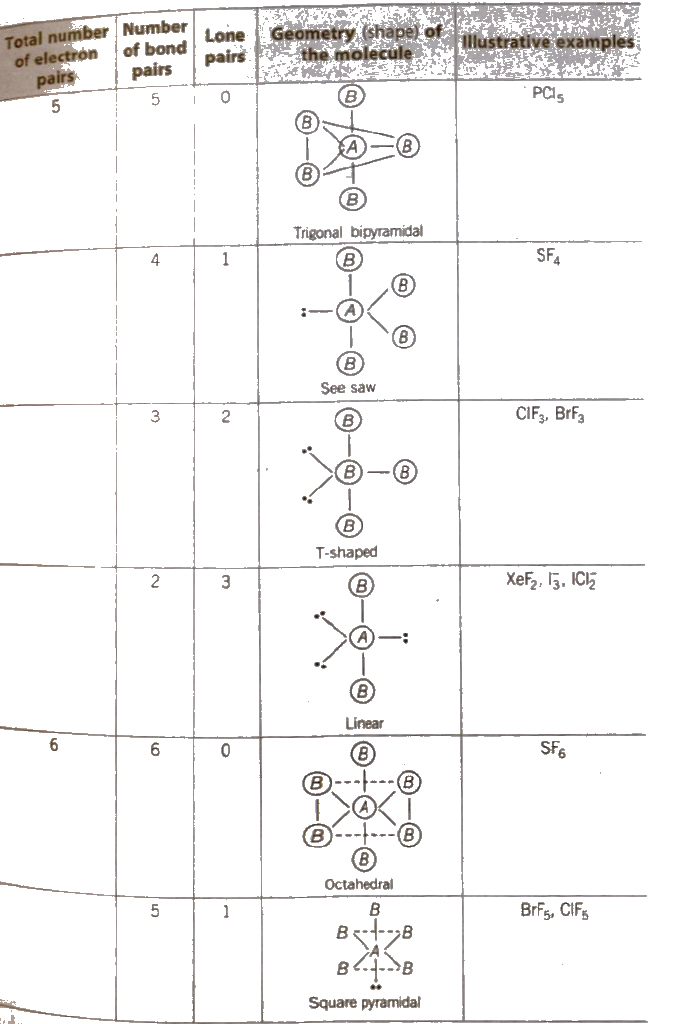
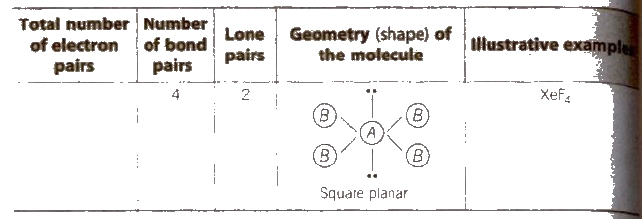
The Valence Shell Electron Pair Repulsion (VSEPR) Theory
According to this theory,
1. The geometry of a molecule or ion depends on the number of electron pairs in the valence shell of its central atom.
2. To attain minimum repulsive state electron pairs try to stay as far away as possible.
3. If the central atom is surrounded by only bonded electron pairs of similar atoms, the repulsive interactions are similar and the moleCular geometry is regular.
4. If the central atom is surrounded by only bonded electron pairs of dissimilar atoms, the repulsive interactions are not equivalent and hence. the geometry of molecule will not be regular.
5. If the central atom is surrounded by both bonded pairs (bp) as well as lone pairs (lp) of electrons. repulsive interactions are not equivalent and hence, geometry of the molecule will be irregular.
The repulsive interactions decrease in the order
lp – lp > lp – bp > bp – bp
Shapes (Geometry) of Molecules Containing Bond Pairs Only or Bond Pairs and Lone Pairs
Valence Bond Theory of Covalent Bond
According to this theory, a covalent bond is formed by the overlapping of two half-filled atomic orbitals having electrons with opposite spins. It is based on wave nature of electron.
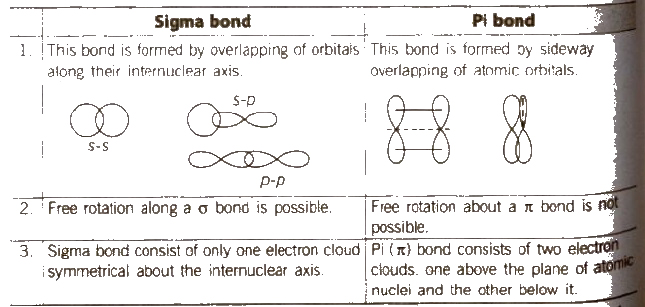
1. Sigma Bond (σ bond)
The following result in the formation of σ bond.
(i) s-s overlapping
(ii) Sop overlapping
(iii) Pop head to head overlapping (axial)
The strength of 0 bond depends upon the extent of overlapping between atomic orbitals. The greater the extent of overlapping, the stronger is the σ bond.
2. Pi Bond (π bond)
It is formed by the sidewise or lateral overlapping between p- atomic orbitals [pop side by side or lateral overlapping]
π bond is a weaker bond than σ bond.
Comparison of Sigma and Pi Bonds
Limitations of VBT
1. The magnetic properties. of some molecules. It fails to explain.
2. Bonding in electron deficient compounds.
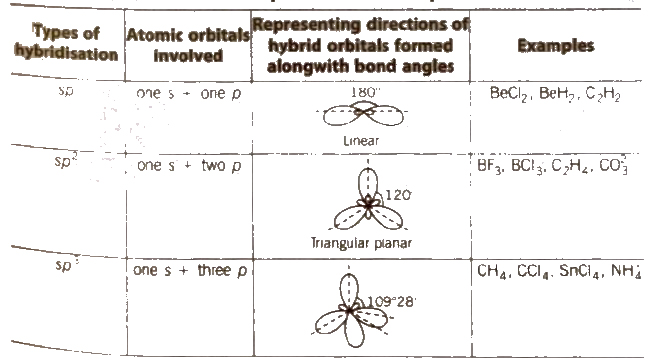
Hybridisation
It is defIned as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new orbital of equal energies and identical shapes. The new orbitals thus formed are known as hybrid orbitals and are more stable,
Method for Finding the Hybridisation
Apply tho following formula to find the hybridisation of central atom.

Examples
Hybridisation of NH3 = 1 / 2[5 + 3 + 0 – 0] = 4 ⇒sp3
Hybridisation of2-4 = 1 / 2[6 + 0 + 2 – 0] = 4 ⇒sp3
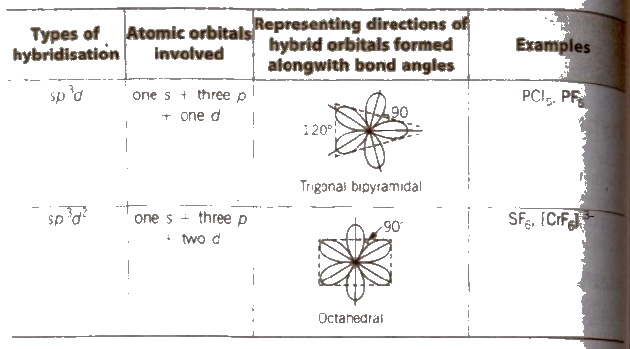
Some Common Types of Hybridisation with Shapes and Examples
Coordinate or Dative Bond
It is a type of covalent bond in which the electron pair (lone pair) is donated by one atom but shared by both the atoms so as to complete their octets. e.g.,

Molecular Orbital Theory
According to this theory, the atomic orbitals combine to form the molecular orbitals. The number of molecular orbitals formed is equal is the number of atomic orbitals involved. According to this theory.
1. The molecular orbitals are formed by LCAO (Linear combination of atomic orbitals) method, i.e., by addition or subtraction of wave functions of individual atoms, thus
ΨMO = ΨA ± ΨB
Ψb = ΨA + ΨB
Ψa = ΨA – ΨB
2. Molecular orbital of lower energy is known as bonding molecular orbital and that of higher energy is known as anti-bonding molecular orbital.
3. Aufbau rule, Pauli’s exclusion principle and Hund’s rule are all applicable for molecular orbitals.
4. The shape is governed by the shape of atomic orbitals, e.g., s-s and p-p overlapping.
(i) Combination between s-atomic orbitals

(ii) Combination between 2s and 2s orbitals gives σ2s and σ 2s orbitals.
(iii) Combination between p-atomic orbitals
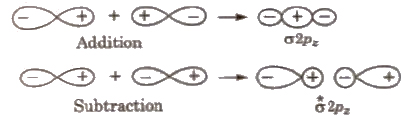
(iv) Combination between 2 px and 2 py atomic orbitals
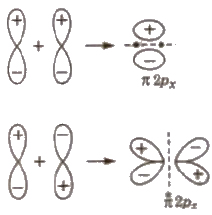
2 py atomic orbitals will also overlap in the same way and thus, resulting molecular orbitals are π 2 py and π 2 py.
If molecular orbital has symmetry with respect to centre, it is called gerade (g) otherwise ungerade (u). All σ bonding and π anti-bonding MO are g while all π bonding and σ anti-bonding MO are u.
Electronic Configuration and Bond Order (BO) Of Molecular
The order of energy of molecular orbitals has been determined experimentally by spectroscopy for the elements of the second period. The increasing order of energies of the molecular orbitals in homonuclear diatomic molecules is
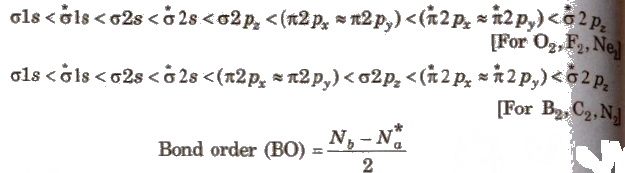
[Molecular species having unpaired electrons are paramagnetic, while if all the electrons in the orbitals are paired then the molecule is diamagnetic.]
Hydrogen Bond
It is defined as the force of attraction existing between hydrogen atom covalently bonded to highly electronegative atom (N, O or F) and the electronegative atom belonging to another molecule of the same or different substance. It is represented by dotted lines. The chains possess a zig – zag structure.
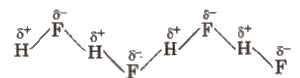
(Hydrogen bond is purely electrostatic and a weak bond. The strength of the strongest hydrogen bond is about 5-10 kcal per mol. The more the electronegativity of atom involved in H-bonding, the more is the bond strength, e.g.,
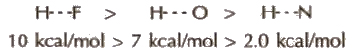
Types of hydrogen bonds are
(i) Intermolecular H-bonding : H-bonding involving two or more molecules.
(ii) Intramolecular H-bonding : H-bonding within a molecule.
Applications of Intermolecular H-bonding
(i) Melting point and boiling point of water Water has the lowest molecular weight among the hydrides of group 16 elements yet it has the highest melting and boiling points. It is due to intermolecular H-bonding in H2 O.
(ii) Ice has less density than water In crystal structure of ice every water molecule is associated with four other water molecules by H-bonding in a cage like tetrahedral structure. On melting, the ice H-bonds are broken and space between water molecules decreases and density of water increases up to 4o C Above 4°C. more H-bonds are broken. the water molecules get apart from each other and the density again decreases. Thus, water has maximum density at 4°C.
(iii) Melting point and boiling point of alcohols The marked difference between the melting and boiling points of alcohols is also due to H-bonding.
Applications of Intramolecular H-bonding
Volatile character of nitrophenols o-nitrophenol is more volatile (b.p. 214°C) as compared to meta (b.p. 290°C) and para (b.p. 279°C). It is due to chelation.

In meta and para isomer chelation is not possible due to the formation of desired size of ring.
Metallic Bond
Metallic bond is the force of attraction between a metal ion to a number of electrons within its sphere of influence. Electron-sea theory of metallic
bond explains number of the properties of the metal
bond explains number of the properties of the metal
Strength of bonds
Ionic bond > covalent bond > metallic bond > H-bond

