Class 11 Chemistry Chapter Equilibrium Notes

Physical processes involve such changes, which only affects the physical properties of the substance undergoing changes but have no effect on the chemical composition and properties.
Chemical processes involve changes in chemical composition and properties. Whenever a chemical change occurs, we can say that a chemical reaction has taken place.
Types of Chemical Reactions
1. Combination Reactions
In such reactions two or more substances combine to form a single compound.
e.g.,
2Mg + O2 → 2MgO
2. Decomposition Reactions
In these reactions. a compound decomposes to produce two or more different substances.
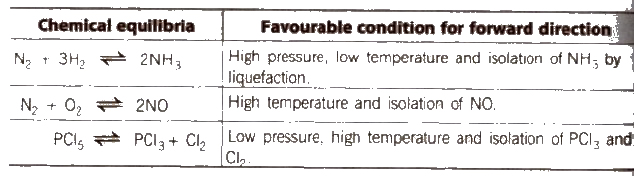
e.g., PCI5 ⇔ PCI3 + CI2
Digestion of food is also a decomposition reaction.
[Decomposition by heat IS called thermal decomposition and decomposition by sunlight is called photo decomposition.]
3. Displacement Reactions
These reactions involve displacement of one element or group by another. These are infact, redox reactions, e.g.,
Zn(s) + H2SO4
4. Double Displacement or Metathesis Reactions
In these. reactions two compounds react to form two new compounds and no change in oxidation state take place, e.g., precipitation reactions, neutralisation, reactions.
AgNO3(aq) + NaCI(aq) → → AgCl(s) + NaNO3(aq)
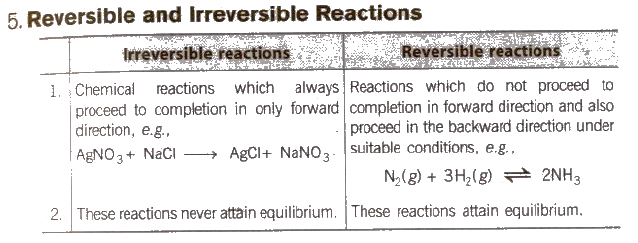
Equilibrium State
Under given set of conditions if a reversible process or chemical reaction is carried out in a closed container, a constancy in some observable properties like colour intensity, pressure, density, is observed. Such a state is referred to as an equilibrium state.
Equilibrium may be classified as :
Physical Equilibrium
Equilibrium set up in physical processes like evaporation of water, melting of solids, dissolution of solutes, etc., is called physical equilibrium, e.g., Ice ⇔ Water
At equilibrium,
Rate of melting of ice = Rate of freezing of water
Chemical Equilibrium
If a reversible reaction is carried out in a closed vessel, a stage is attained where the speed of the forward reaction equals the speed of the backward reaction. It corresponds to chemical equilibrium. At equilibrium,
Rate of forward reaction = Rate of backward reaction
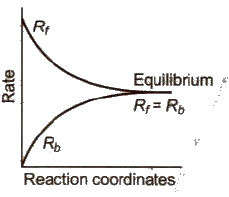
Characteristics of Chemical Equilibrium
1. Equilibrium can be attained from either side.
2. Equilibrium is dynamic in nature, i.e., at equilibrium reaction does not stop.
3. At equilibrium, there is no change in the concentration of various species.
4. The equilibrium state remains unaffected by the presence of catalyst. Catalyst helps to attain the equilibrium state rapidly.
6. The observable physical properties of the process become constant.
Law of Mass Action
Guldberg and Waage states that the rate of a chemical reaction is directly proportional to the product of the active masses of the reacting substances. For a general reaction,
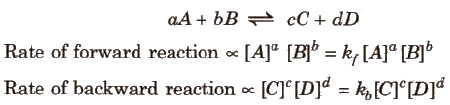
where, kf and kb are rate constants.
In heterogeneous equilibrium, the active mass of pure solids and liquids are taken as
At equilibrium,
Rate of forward reaction = Rate of backward reaction
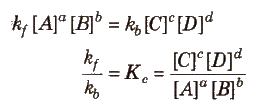
Kc is called the equilibrium constant.
Use of Partial Pressures Instead of Concentration
For gaseous reactions, partial pressures are conveniently used since at any fixed temperature partial pressure is directly proportional to concentration. For a general gaseous reaction,
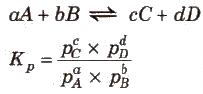
Relation between Kc and Kc
where, Δng = moles of products – moles of reactants (gaseous only)
Relation between Kc and Kp for different types of reactions
(i) When Δng = 0, Kp = Kc
(ii) When Δng = +ve, Kp > Kc
(iii) When Δng = -ve, Kp < Kc
Units of Kp and Kc
(i) Unit of Kp = (atm)Δng
(ii) Unit of Kc = (mol L-1)Δng
Characteristics of Equilibrium Constant Kp or Kc
1. It has definite value for every chemical reaction at a particular temperature.
2. The more is the value of Kc or Kp, the more is the extent of completion of reaction, i.e., Kc < 1 indicates lesser concentration of products than reactants.
K ≥ 103 shows completion of reaction and K ≤ 10-3 shows that the reaction does not proceed at all.
3. When the reaction can be expressed as sum of two other reactions, the Kc of overall reaction is equal to the product of equilibrium constants of individual reactions.
4. The equilibrium constant is independent of initial concentrations of reactants.
5. Equilibrium constant is independent of presence of catalyst.
6. Kc for backward reaction is inverse of Kc for forward reaction.
7. If an equation is multiplied by n, the K becomes Kn, and if it is divided by m, the k becomes m √k.
8. In equilibrium constant expression if activities are used in places of molar concentration, h becomes dimensionless.
Types of Equilibrium
Homogeneous Equilibrium
In homogeneous equilibrium, the reactants and products arc present in the same phase or physical suite (gaseous or liquid).
2SO2(g) + O2(g) ⇔ 2SO3(g)
Heterogeneous Equilibrium
In heterogeneous equilibrium the reactants and products are present in two or more physical states or phases.
3Fe(s) + 4H2O(g) ⇔ Fe3O4(s) + 4H2(g)
Reaction Quotient
For any reversible reaction at any stage other than equilibrium, the ratio of the molar concentrations of the products to that of the reactants. where each concentration term is raised to the power equal to the stoichiometric coefficient to the substance concerned, is called the reaction quotient, Qc.
For a general reaction
aA + bB ⇔ cC + dD
which is not at equilibrium,
Qc = [C]c + [D]d / [A]a [B]b
If
(i) Qc > Kc, the value of Qc will tend to decrease to reach the value of Kc(towards equilibrium) and the reaction will proceed in the reverse direction.
(ii) Qc < Kc it will lend to increase and the reaction will proceed in the forward direction.
(ii) Qc = Kc, the reaction is at equilibrium.
Le – Chatelier’s Principle
There are three main factors which affect the state of equilibrium.
They are
- concentration
- temperature
- pressure.
Le – Chatelier’s principle states that if a system at equilibrium is subjected to a change in concentration. pressure or temperature. the equilibrium equilibrium change.
Effect of Change of Concentration
If at equilibrium the concentration of one of the reactants is increased. the equilibrium will shift in the forward direction and vice-versa.
Effect of Change in Pressure
No effect of pressure on equilibria having same moles of reactants and products. e.g., N2 + O2 ⇔ 2NO.
When there is change in the number of moles, the equilibrium will shift in the direction having smaller number of moles when the pressure is increased and vice-versa, e.g.,
N2 + 3H2 ⇔ 2NH3 [High p. high yield of NH3]
Effect of Temperature
When process is exothermic, low temperature favours the forward reaction. When process is endothermic. high temperature favours the formation of products.
Effect of Addition of Inert Gas
(i) Addition of inert gas at constant pressure At constant pressure. if an inert gas is added. it will increase the volume of the system. Therefore. the equilibrium will shift in a direction in which there is an increase in the number of moles of gases.
(ii) Addition of inert gas at constant volume If keeping volume of the system constant, an inert gas is added. the relative molar concentration of the substance will not change. Hence. the equilibrium position of the reaction remains unaffected.
Effect of Catalyst
The presence of catalyst does not change the position of equilibrium. It simply fastens the attainment of equilibrium.
Le-Chatelier’s Principle Applicable to Physical Equilibrium
(i) Effect of pressure on solubilityThe increased pressure, will increase the solubility of gas and vice-versa.
(ii) Effect of temperature on solubility Some substances dissolve with the absorption of heat. Solubility of such substances will increase with increase of temperature and vice-versa, e.g., dissolution of NH4CI, KCI, KNO3, etc. The dissolution of calcium acetate and calcium hydroxide is exothermic, so their solubility is lowered at higher temperature.
(iii) Effect of pressure on the melting point of ice
Ice ⇔ liquid water
The ice occupy the more volume than liquid water, so increased pressure will result in melting of ice according to Le-Chatelier principle.
Favourable conditions for some chemical equilibria to get higher yield of product.
Calculation of the Degree of Dissociation (α) from Density Measurement
α = D – d / d
where, D = theoretical vapour density
d = observed vapour density
Now, molecular mass = 2 * VD
∴ α = Mc – Mo / Mo
where, Mc = calculated molecular weight
Mo = observed molecular weight

