Class 11 Chemistry Chapter Hydrocarbons Notes

Classification of Hydrocarbons
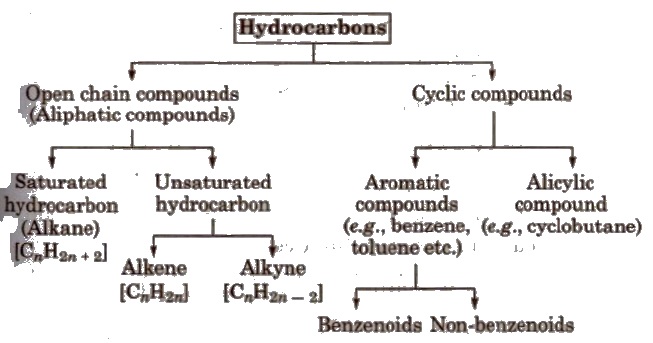
Alkanes
Alkanes are saturated, open chain hydrocarbons containing carbon-carbon single bonds. e.g., methane (CH4), ethane (C4H6) propane (C3H8), etc.
These hydrocarbons are inert under normal conditions [i.e.,do not react with acids. bases and other reagents). Hence, they were earlier known 88 paraffins (Latin : parum-little; affins-affinity)
Alkanes exhibit chain isomensm, position isomerism and conformational isomerism.
Methods of Preparation of Alkanes
i) From hydrogenation of alkenes and alkynes
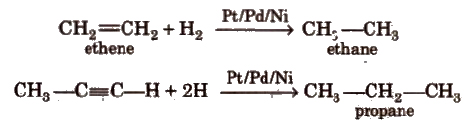
Ease of hydrogenation depends on the steric crowding across the multiple bond. More is the steric crowding, the less is the
reactivity towards hydrogenation.
reactivity towards hydrogenation.
(ii) By sodalime Decarboxylation of sodium or potassium salts of fatty acids [decarboxylation reaction]
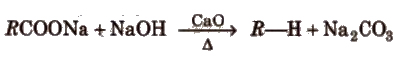
This reaction is used for descending of series as the alkane obtained has one carbon less than the parent compound. CaO is
more hygroscopic than NaOH and it keeps NaOH in dry state.
more hygroscopic than NaOH and it keeps NaOH in dry state.
(iii) By Wurtz reaction
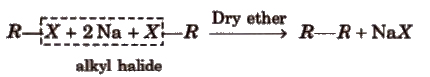
This reaction is used to increase the length of the carbon chain.
(iv) By reduction of alkyl halides
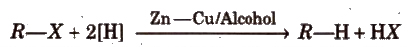
Reducing agents like Zn/HCl, HI/Red P, H2/Pd can also be used.
(v) By Kolbe’s electrolysis
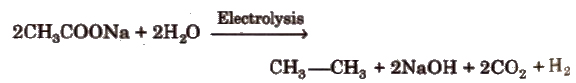
Only alkanes with even number of carbon atoms can be formed.
Alkane and CO2 are liberated at anode while H2 is liberated at cathode.
(vi) Clemmensen’s reduction
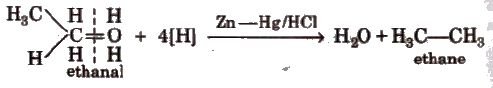
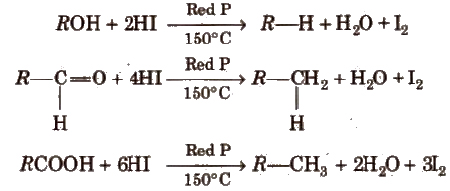
(vii) From compounds containing oxygen Alcohols, aldehydes ketones, carboxylic acids and their derivatives give alkane when treated with hot conc HI and red P in a sealed tube.
(viii) Wolff-Kishnerts reduction

(ix) From carbides

(x) Corey-Bouse synthesis This method can be used to prepare alkanes having odd number of carbon atoms.
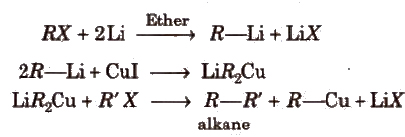
Physical Properties of Alkanes
(i) The first four members are colourless gas, next thirteen members are colourless liquids and next higher members are
colourless solids.
colourless solids.
(It can be explained on the basis of magnitude of attraction forees.)
(ii). Boiling point of alkanes decreases on branching.
BP ∝ VAF (van der Waals’ forces)
VAF ∝ molecular mass or VAF ∝ SA (surface area)
So boiling point order can be given as
n-octane > iso-octane> 2, 2, 3, 3-tetramethyl butane
(iii). Alkanes with even number of carbon atoms have higher melting points as compared to next higher or lower alkanes with odd number of carbon atoms.
(iv). Alkanes being non-polar in nature, soluble in non-polar solvents but insoluble in polar solvent such as water.
Chemical Properties of Alkanes
(i) Halogenation of alkanes
(a) Chlorination
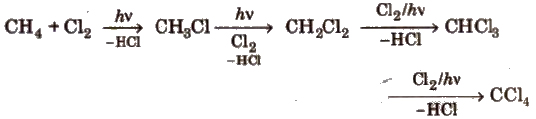
(b) Bromination of alkanes proceeds in the same way but not so easily.
(c) Iodination
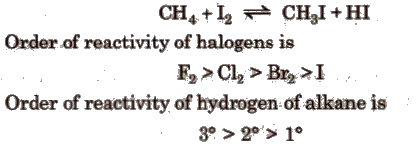
Mechanism of halogenation of alkanes is free radical in nature, i.e., the attacking reagent is a halogen free radical (X.). It is a chain reaction.
(ii) Combustion
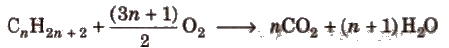
Due to the evolution of a large amount of heat during combustion, alkanes are used as fuels.
(iii) Controlled oxidation
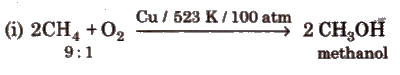

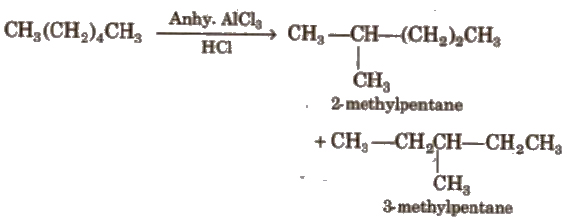
(iv) Isomerisation
(v) Aromatisation
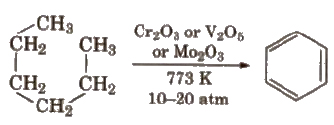
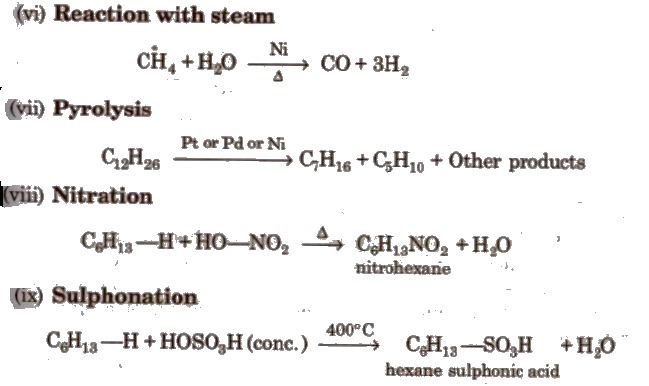
Reactions for Methane (CH4)
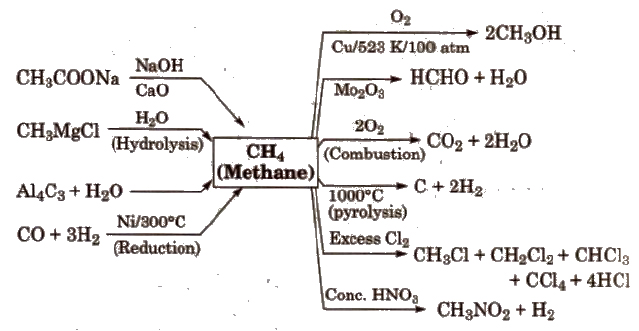
(Methane cannot be prepared by Wurtz reaction, Kolbe’s electrolytic process and by reduction of alkenes or alkynes).
Reactions for Ethane(C2H6)
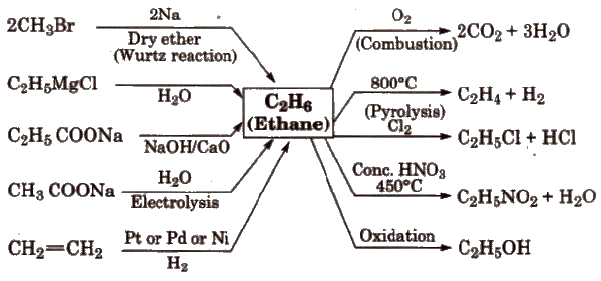
Conformations of Alkanes
Alkanes have C-C sigma (σ) bonds and rotation about C-C single bond is allowed. This rotation results in different spatial arrangements of atoms in space which can change into one another, such spatial arrangements are called conformations or conformers or rotamers.
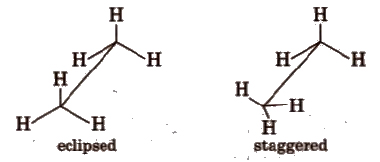
Conformations of ethane
(i) Sawhorse projections
(ii) Newman projections
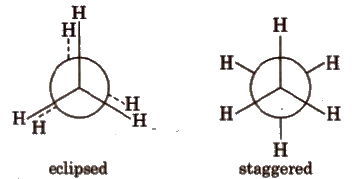
Intermediate conformation between eclipsed and staggered are known as skew (gauche) conformations.
Eclipsed form is least stable but staggered form is most stable due to greater distance between the bond pairs or lesser torsional strain.
The energy difference between the two extreme forms is of the order of 12.5 kJ mol-1.
Alkenes
These are unsaturated non-cyclic hydrocarbons which have.sp2 -hybridisation with 120° bond angle.
Alkenes are also called olefins [oil.forming] which indicates their high reactive nature.
Alkenes have general formula CnH2n, where n = 2,3,4 …
C2H4 (ethene), C3H6 (propene), etc.
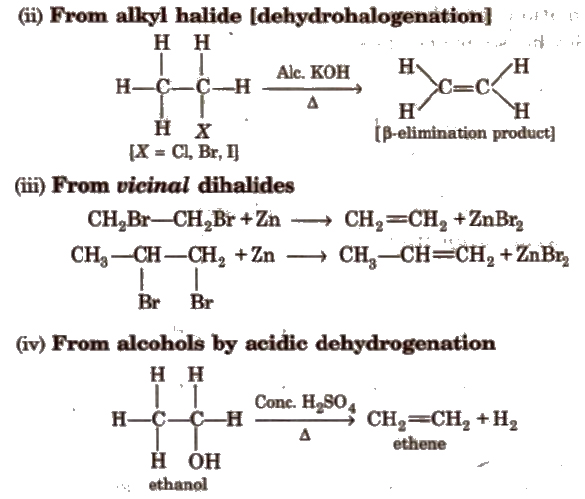
Methods of Preparation of Alkenes
(i) From aIkynes
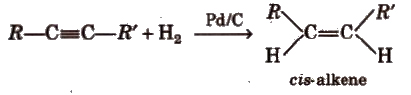
Physical Properties of Alkenes
Alkene as a class resemble alkanes in physical properties, except in types of isomerism and difference in polar nature.
C1 to C3 are gases, the next fourteen are liquids and the higher members are solids.
Alkenes show a regular increase in boiling point with increase in size.
Alkenes show a regular increase in boiling point with increase in size.
Isomerism in Alkenes
Alkene show both structural isomerism and geometrical isomerism.
Structural isomerism exhibited by alkenes are chain isomerism and position isomerism.
Alkenes also exhibit stereoisomerism as geometrical (cis-trans) isomerism.

Isomerism in Alkenes
Chemical Properties of Alkenes
(i) Addition of halogens
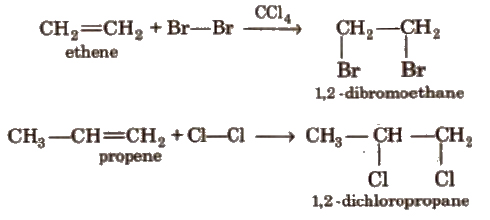
(ii) Addition of hydrogen halides HCI, HBr, HI add up to alkenes to form alkyl halides as per their reactivity order
HI > HBr > HCI
Addition reaction of HBr to unsymmetrical alkenes (Markownikoff’s rule) According to Markownikofrs rule, the negative part of the addendum (adding molecule) gets attached to that carbon atom which possesses lesser number of hydrogen atom.
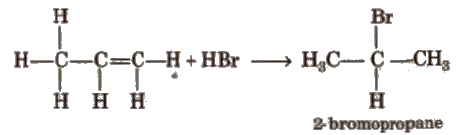
Anti-Markownikoff addition or peroxide effect or kharash effect In the presence of organic peroxide, addition of only HBr molecule on unsymmetrical alkene takes place contrary to the Markownikoffs rule.
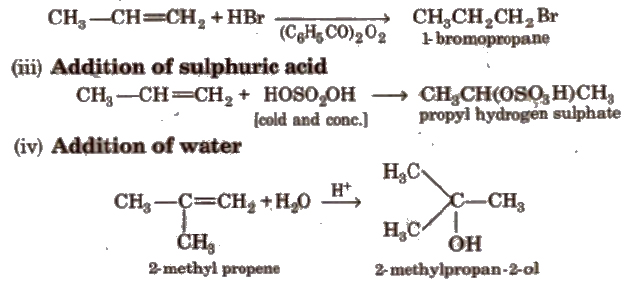
(v) Oxymercuration-demercuration This reaction is an example of hydration of alkene according to Markownikoffs rule.
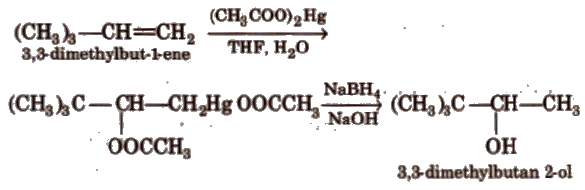
It is an anti-addition reaction.
It is better than catalytic hydration by dil. H2SO4, as it avoids rearrangement.
(vi) Bydroboration oxidation
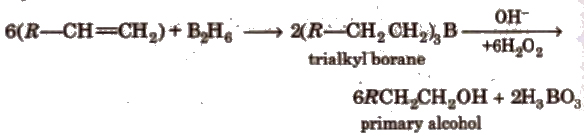
This reaction involved syn-addition of reagent.
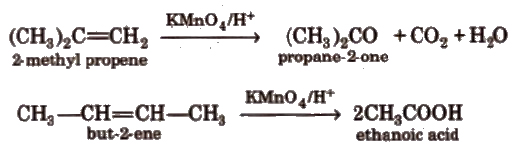
(vii) Oxidation Alkenes decolourise cold dilute aqueous solution of potassium permanganate (Baeyer’s reagent). It is used as a test for unsaturation.
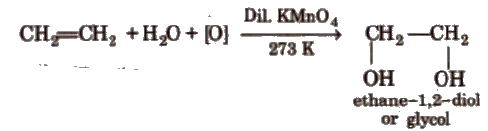
Acidic KMnO4 or acidic K2Cr2O7oxidise alkenes to ketones and/or acids depending upon the nature of alkene and the experimental conditions.
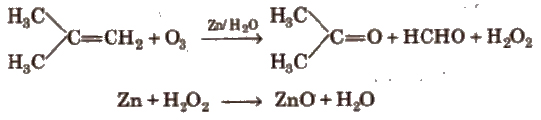
(viii) Ozonolysis
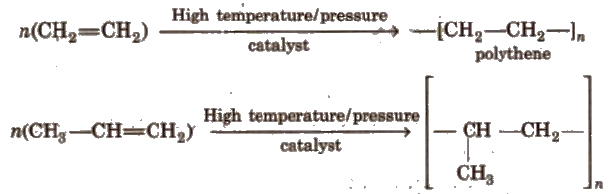
(ix) Polymerisation
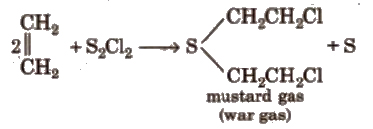
(x) Reaction with sulphur monochloride
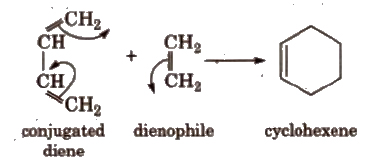
(xii) Diels-Alder reaction
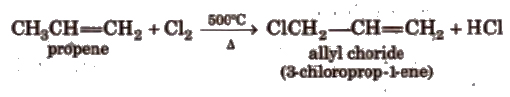
(xiii) Substitution reactions These occur at very high temperature at allylic position
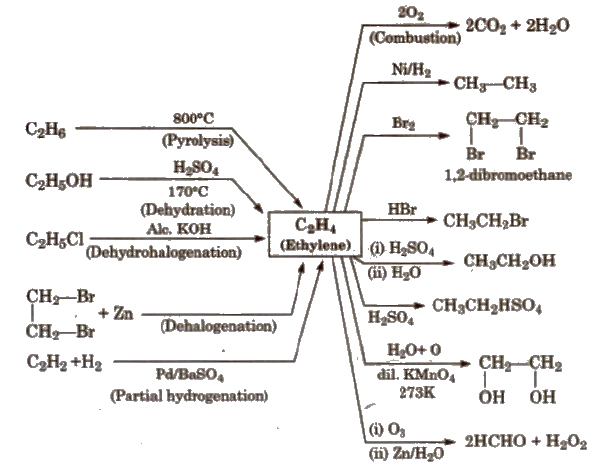
Reactions for Ethene [Ethylene] (C2 H4)
Conjugated dienes
Dienes having alternate single (-) and double bonds (=) are called conjugated alkenes. These give Diels, Alder reaction.
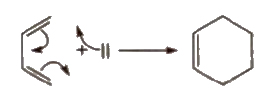
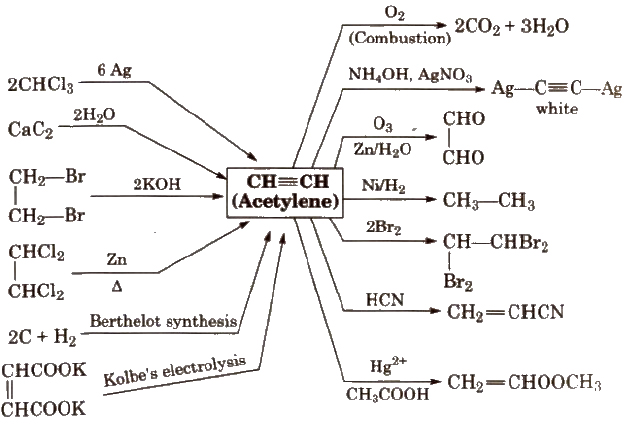
Alkynes
These are unsaturated hydrocarbons with general formula CnH2n – 2 e.g., C2H2 (ethyne), C3H4(propyne)
Alkynes also exhibit electrophilic addition reaction but less reactive than alkenes because the dissociation of x-electron cloud requires more energy.
In alkynes, position of triple bond is determined by ozone (O3).
H – C = C – H contains 3σ and 2π – bonds and bond length is 120 pm. In acetylene. H – C – C bond angle is 180°.
Methods of Preparation of Alkynes
(i) From calcium carbide
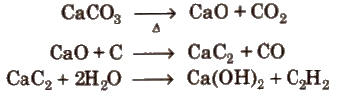
(ii) From vicinal dihalides
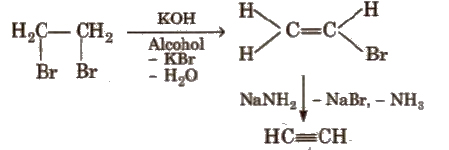
(iii) From tetrahalides
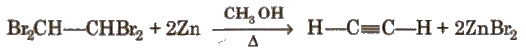
Physical Properties of Alkynes
1. The rust two members are gases next eight members (C5 – C12) are liquids and higher members are solids.
2. They are all colourless and odourless with the exception of acetylene which has slightly garlic odour due to the presence of PH3and H2S as impurities.
2. They are all colourless and odourless with the exception of acetylene which has slightly garlic odour due to the presence of PH3and H2S as impurities.
3. Alkynes are insoluble in water but soluble in organic solvents like ethers, carbon tetrachloride and benzene.
4. Melting point, boiling point and density increase with increase in molar mass.
Chemical Properties of Alkynes
Alkynes show electrophilic as well as nucleophilic addition reactions.
(i) Acidic character of alkyne
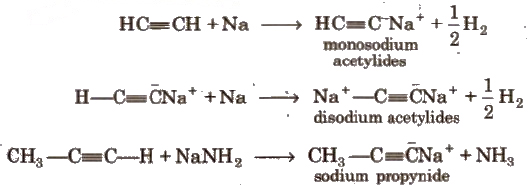
These reactions are not shown by alkenes, alkanes and non-terminal alkynes, hence used for distinction between alkane, alkene and alkyne.
Acetylenic hydrogens are acidic in nature due to 50% a-character in sp-hybridised orbitals.
Acidity of alkynes is lesser than water.
Acidic behaviour order
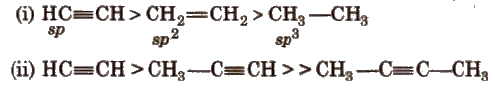
(ii) Electrophilic addition reactions
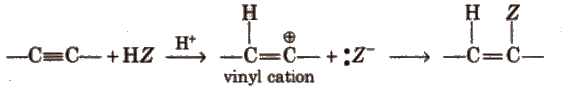
The addition product formed depends upon the stability of vinylic cation. Addition on unsymmetrical alkynes takes place according to Markovnikov’s rule.
(a) Addition of dihydrogen

(b) Addition of halogens
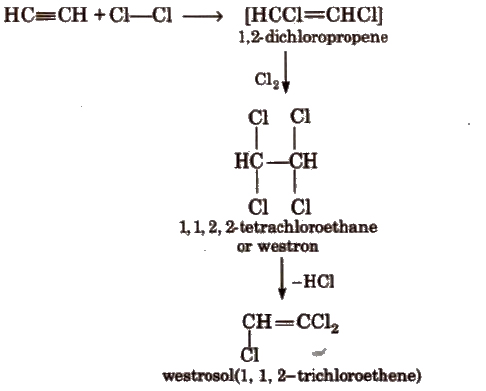
(c) Addition of hydrogen halides
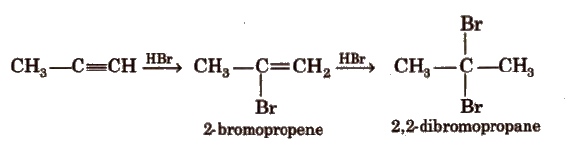
(d) Addition of water
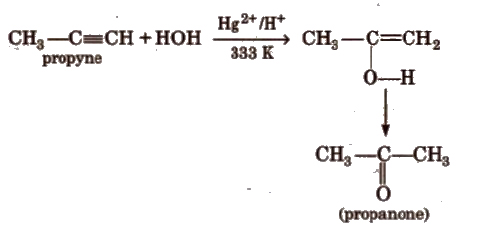
(iii) Cyclic polymerisation
(iv) Reaction with AsCl3 (arsenic trichloride)

(v) Oxidation
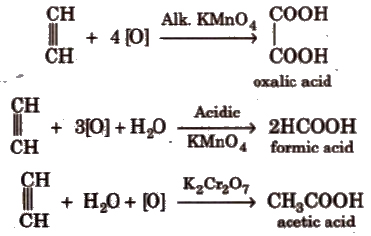
Ozonolysis
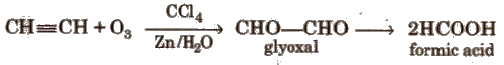
Higher alkynes give diketones which are further oxidised to carboxylic acid.
(vi) Linear polymerization

Reactions for Acetylene (C2 H2)
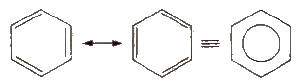
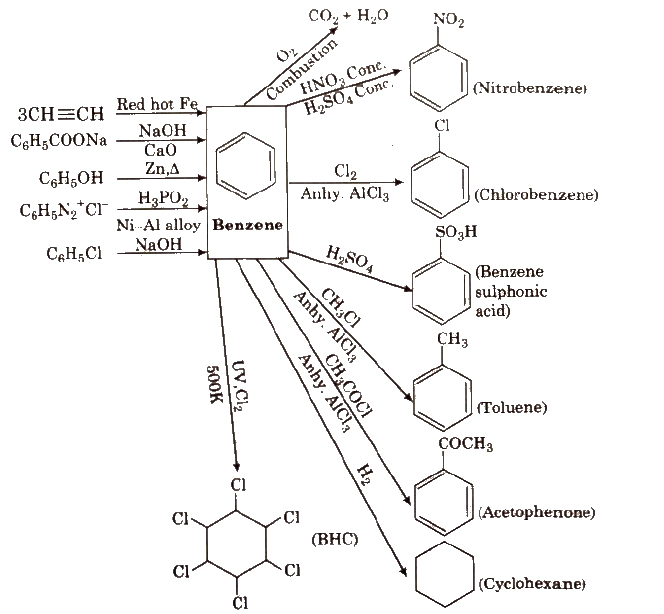
Benzene
The parent member of the family of aromatic hydrocarbons is benzene (molecular formula: CJls). It has hexagonal ring of six carbon atoms with three double bonds at alternate positions. It is resonance stabilised and the structure may be represented as given ahead.
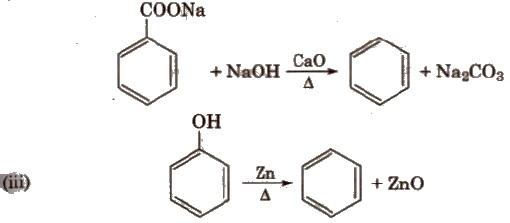
Methods of Preparation
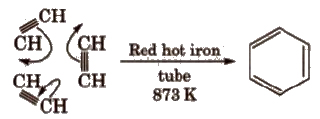
(i) Cyclic polymerisation of ethyne See alkyne
(ii) Decarboxylation of aromatic acids
Physical Properties of Benzene
Aromatic hydrocarbons are non-polar molecules and are usually colourless liquids or solids with a characteristic aroma.
Aromatic hydrocarbons are immiscible with water but readily miscible with organic solvents.
Aromatic compounds burn with sooty flame.
Chemical Reactions of Benzene
Benzene gives electrophilic substitution reactions.
According to experimental evidences, electrophilic substitution reaction involve following three steps:
(a) Generation of electrophilic
(b) Formation of carbocation intermediate
(c) Removal of proton from the carbocation intermediate.
(b) Formation of carbocation intermediate
(c) Removal of proton from the carbocation intermediate.
(i) Nitration
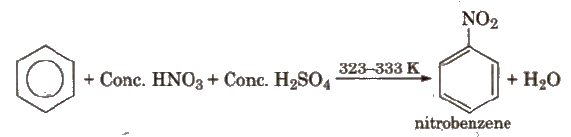
(ii) Halogenation
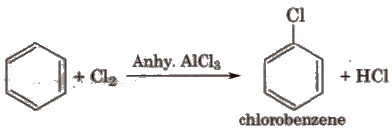
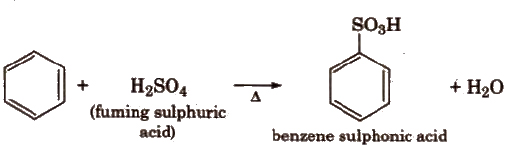
(iii) Sulphonation
(iv) Friedel-Craft’s alkylation reaction
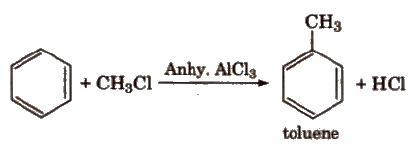
When Friedel-Craft alkylation is carried out with CH3CI, the product obtained is C6H5CH3. Incase the alkylation is carried out
with higher alkyl halide, e.g., n-propyl chloride, then the electrophile n-propyl carbocation (CH3 – CH2 – +CH2) which is a
primary carbocation rearranges to form more stable secondary carbocation (iso-propyl carbocation)., and the main product formed will be iso-propyl benzene.
with higher alkyl halide, e.g., n-propyl chloride, then the electrophile n-propyl carbocation (CH3 – CH2 – +CH2) which is a
primary carbocation rearranges to form more stable secondary carbocation (iso-propyl carbocation)., and the main product formed will be iso-propyl benzene.
(v) Friedel-Crafts acylation reaction
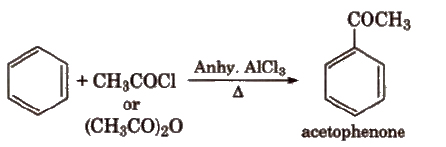
(vi) With Cl2 In excess of chlorine, benzene yields hexachlorobenzene [C6CI6].
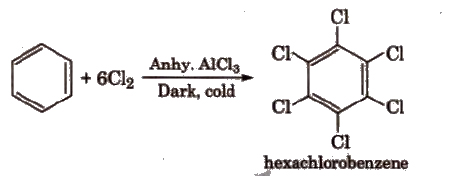
Benzene also undergoes addition reactions e.g.,
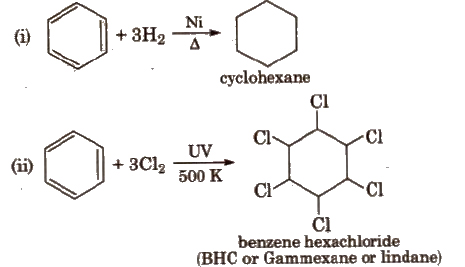
Combustion 2C6H6 + 15O2 → 12CO2 + 6H2O
Reactions for Benzene
Petroleum
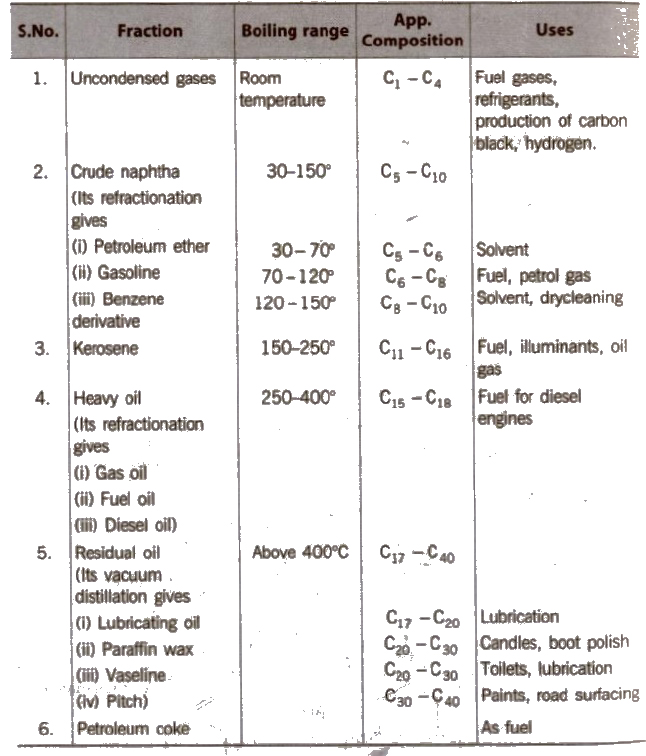
It is a dark coloured oily liquid with offensive odour, found at various depths in many region below the earth’s surface. It is also called rock oil, mineral oil or crude oil It is covered by an atmosphere of a gaseous mixture known as natural gas.
It contains mainly alkanes, cycloalkanes, aromatic hydrocarbons, sulphur, nitrogen and oxygen compounds.
When subjected to fractional distillation, it gives different fractions at different temperatures.
LPG (Liquified Petroleum gas)
It is a mixture of butane and isobutane with a small amount of propane. A strong foul smelling substance, called ethyl mercaptan (C2H5SH) is added to LPG cylinders, to help in the detection of gas leakage.
CNG (Compressed Natural Gas)
It consists mainly of methane (95%), which is a relatively unreactive hydrocarbon and makes its nearly complete combustion possible.
Artificial Methods for Manufacturing Petrol
From higher alkanes, petrol or gasoline is obtained by cracking or pyrolysis.
From coal, petrol can be synthesised by following two processes :
(i) Bergius process

(ii) Fischer- Tropsch process
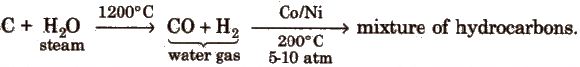
The best catalyst for this process is a mixture of Co, thoria, magnesia and kieselguhr.
The overall yield in this process is slightly higher than Bergius process.
Octane Number
The quality of petrol is expressed in terms of octane number which is defined as the percentage of iso-octane by volume in a mixture of iso-octane and n-heptane which has the same antiknock properties as the fuel under test.
The octane number is 100 for iso-octane (2, 2, 4-trimethylpentane)
Natural gas has octane number 130.
TEL (tetraethyl lead) is used as antiknocking compound.
Octane number is increased by isomerisation, alkylation or aromatisation.
Cetane Number
Quality of diesel oils is measured in terms of cetane number which is defined as the percentage of cetane (hexadecane) by volume in a mixture of cetane and a-methyl naphthalene which has the same ignition property as fuel oil under similar experimental conditions.
It is 100 for cetane and 0 for α – methyl naphthalene.

