Class 11 Chemistry Chapter Hydrogen Notes

Position of Hydrogen in the Periodic Table
Hydrogen resembles with alkali metals (group I) as well as halogens (group 17), At the same time, it differs from both in certain characteristics. That is why hydrogen if; called “rogue element”.
However. it has been placed in group 1 on the basis of its configuration 1s1, which is the basis of modern classification of elements.
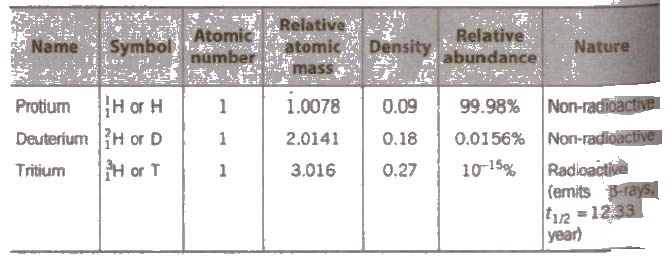
Isotopes of Hydrogen
Hydrogen exists in the form of three sotopes :
Dihdrogen [H2]
Methods of Preparation
(a) Lab pletbods

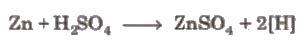
Metals which have reduction potential lesser than H, can liberate H2 from acids.
Pure zinc is not used because it reacts slowly. The presence of some impurities increases the rate of reaction due to the
formation of electrochemical couples Cone sulphuric acid is also not used because it oxidises, H2formed into H2O.
formation of electrochemical couples Cone sulphuric acid is also not used because it oxidises, H2formed into H2O.
Zn + 2H2SO4(conc.) → ZnSO4 + SO2+ 2H2O
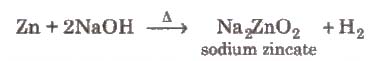
(ii) It can also be prepared by the reaction of zinc with aqueous alkali.
b) Commercial production of dihydrogen
(i) By the electrolysis of acidified water
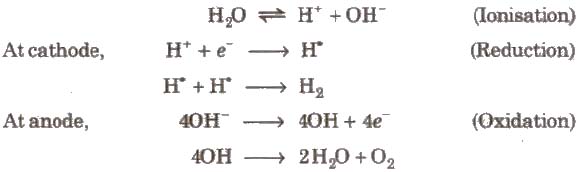
ii) From water gas (Bosch process)
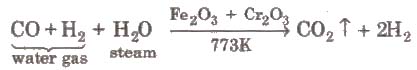
Carbon dioxide is removed by dissolving it in water under pressure (20-25 atm) and hydrogen left behind is collected.
(iii) From steam (Lane’s process) Super heated steam is passed over iron filings heated to about 1023-1073 K when hydrogen is
formed.
formed.
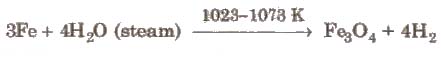
(iv) Highly pure (> 99.95%)dihydrogen is obtained by electrolysing warm aqueous barium hydroxide solution between nickel
electrodes.
electrodes.
(v) From hydrocarbons by partial oxidation
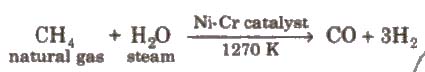
vi) It is also obtained as a by-product in the manufacture of NaOH and chlorine hy the electrolysis of brine solution.
During electrolysis, the reactions that take place are
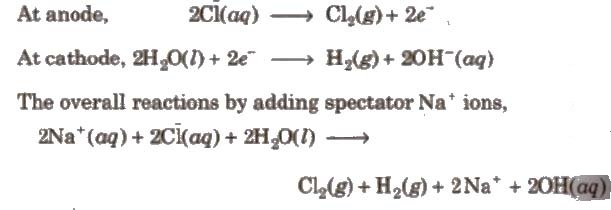
Physical Properties of Dihydrogen
Dihydrogen is a colourless, odourless, tasteless, combustible gas. It is lighter than air and insoluble in water. It is neutral to litmus.
Chemical Properties of Dlhydrogen
(i) Reactivity The relative inertness of dihydrogen at room temperature is because of Its high enthalpy of H-H bond i.e.. high bond dissociation energy. So its reactions take place under specific conditions only (at high temperature).
(ii) Action with non-metals

(iii) Reaction with metals Here H2acts as oxidising agent.
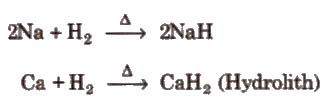
(iv) Reducing action of dihydrogen
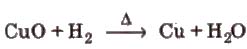
(v) Reactions with metal ions and metal oxides

(vi) Reaction with organic compounds

Uses of Dihydrogen
- It is used in the manufacture of CH3OH.

- It produces temperature of 2850°C and oxy-atomic hydrogen flame produces a temperature of 4000°C, so it is used in oxy-hydrogen flame.
- The largest single use of H2 is in the synthesis of NH3 which is used in the manufacture ofHNO3 and fertilizers.
- Liquid hydrogen (LH2) is used as rocket fuel.
- H2 is used as a reducing agent in extraction of metals.
- H2 is used in fuel cell for generating electrical energy.
- Hydrogen is used in the manufacture of synthetic petrol.
(By heating H2 with coal and heavy oils under very high pressure in the presence of catalyst.)
Different Forms of Hydrogen
Atomic Hydrogen

It is very reactive and its half-life period is 0.33 s.
Nascent Hydrogen
Freshly prepared hydrogen is known as nascent hydrogen and is more reactive than ordinary hydrogen. It causes the reduction of certain compounds which IS not possible with ordinary hydrogen. It can never be isolated.
Activity of nascent H depends upon the reaction by which it is obtained.
Adsorbed Hydrogen
Adsorption of hydrogen at the metal surface is called occlusion. This hydrogen brings out many chemical changes such as reduction and hydrogenation. Occlusion decreases with rise in temperature.
Ortho and Para Hydrogen
When in hydrogen molecule, the nuclear spins are in the same direction, it is known as ortho hydrogen. On the other hand when the nuclear spins arc in tho opposite direction. it is known as para hydrogen. At room temperature hydrogen consists of 75% ortho and 25% para hydrogen.
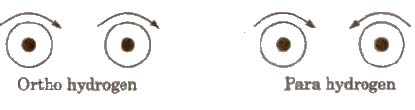
Hydrogen Economy
Hydrogen economy is the use of liquid hydrogen as an alternate source of energy. The technology involves the production, transportation and storage of energy in the form of liquid hydrogen. Large scale production of hydrogen can be done by electrolysis of water or by thermochemical reaction cycle. Storage of hydrogen in liquid form can be done in vacuum insulated cryogenic tanks or in a metal or in all alloy like iron-titanium alloy as interstitial hydride. Hydrogen fuel has many advantage over conventional fuels in that it is non-polluting and it liberates large amount of energy on combustion.
Pbotohydrogen is used to obtain renewable energy from sunlight by using microscopic organism such as bacteria or algae.
Hydrides
The compounds of hydrogen with metals and non-metals are called hydrides.
Ionic Hydrides
These are formed by elements of group I, II, (except Be and Mg) by heating them in hydrogen.
These are white colourless solids (crystalline) having high m.p. and b.p. easily decomposed by water, CO2 or SO2.
CaH2 + 2H2O → Ca(OH)2 + 2H2
CaH2 + 2CO2 → (HCOO)2Ca
They are strong reducing agents. Alkali metal hydrides are used for making LiAlH4, NaBH4, etc and for removing last traces of water from organic compounds.
Molecular or Covalent Hydrides
These are formed by elements of p-block having higher electronegativity than hydrogen.
- Electron deficient hydridesThese are the hydrides which do not have sufficient number of electrons needed to form normal covalent bonds, e.g., BH3, AlH3, etc.
- Electron precise hydridesThese are the hydrides which have exact number of electrons needed to form normal covalent bonds. e.g. hydrides of group 14 (CH4, SiH4, etc.)
- Electron rich hydrides These are the hydrides which have greater number of electrons than required to form normal covalent bonds. e.g., hydrides of group 15, 16, 17, (NH3, PH3 ,H2S, HF, HCl, etc). The excess electrons in these hydrides are present as lone pairs of electrons.
Metallic or Interstitial Hydrides
The transition metals and rare earth metals combine with hydrogen to from interstitial hydrides. They exhibit metallic properties and are powerful reducing agents. They are non-stoicluometric hydrides and their composition varies with temperature and pressure for e.g., LaH2.76, TiH1.73.
Metals of group 7, 8 and 9 do not form hydrides and this region of the Periodic Table is called hydride gap.
Polymeric Hydrides and Complex Hydrides
Polymeric hydrides are formed by’ elements having electronegativity in the range 1.4 to 2.0, e.g., (BeH2)n, (AlH3)n, etc. In complex hydrides H–acts as ligand and is attached to central metal atom, e.g., LiAlH4, LiBH4, etc.
Water
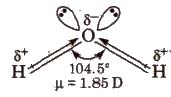
Water is the most abundant and widely distributed on the earth. It occurs in all the three physical states. H2O is a covalent molecule in which oxygen is sp3 hybridised. It has bent structure.
Physical Properties of Water
- Water is a colourless, odourless, tasteless liquid. It has abnormally high b.p., f.p., heat of vaporisation due to hydrogea bonding.
- Pure water is not a good conductor so it is made conductor by adding small amount of acid or alkali.
- Density of ice (which is mass per unit volume) is lesser than that of water and it floats over water.
- Water has maximum density at 4°0.
- Water is a highly polar solvent with high dielectric constant 78.39. It interacts with polar or ionic substances effectively with the release of considerable amount of energy due to ion dipole interaction. The dissolution of covalent compounds like urea, glucose and C2H5OH, etc is due to the tendency of these molecules to form hydrogen bond with water.
Chemical Properties of Water
1. Water is amphoteric in nature.
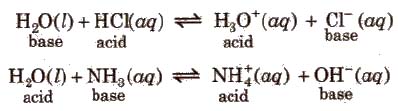
2. In redox reactions,water reacts with metals and non- metals both.
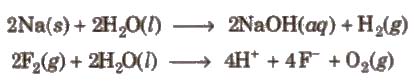
3. In hydrated salts, water may remain in five types such as coordinated water, hydrogen bonded water, lattice water, clathrate water and zeolite water.
4. A number of compounds such as calcium hydride, calcium phosphide. etc ., undergo hydrolysis with water.
Purification of Water
It involves two processes
- Removal of suspended impurities
- Destroying the bacteria.
Suspended particles are removed by coagulation with alum followed by filtration.
Exposure to sunlight, boiling, chlorination (treatment with liquid Cl2 or bleaching powder), ozonisation and addition of CuSO5are some processes which are employed to destroy bacteria.
Heavy Water [D2O]
It was discovered by Urey in 1932. It can be prepared by exhaustive electrolysis of ordinary water using nickel electrodes. It is colourless, odourless, tasteless liquid.
Chemical Reactions of Heavy Water
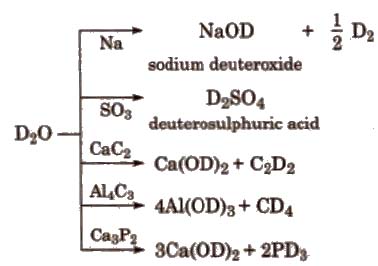
Usesof Heavy Water
It is used
- in nuclear reactors to slow down the speed of neutrons and called moderator.
- as a tracer compound to study the mechanisms of many reactions.
Soft and Hard Water
The water which produces large amount of lather with soap is known as soft water and which forms a scum with soap is known as hard water.
Typesof Hardness of Water
- Temporary hardness It is due to the presence of bicarbonates of calcium and magnesium.
- Permanent hardness Tt is due to the presence of chlorides and sulphates of calcium and magnesium.
Removal of Temporary Hardness
It can be achieved:
(a) By boiling The soluble bicarbonates are converted into insoluble carbonates.
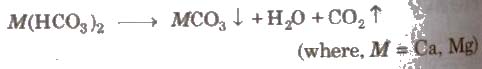
(b) By Clark’s process By adding lime water or milk of lime.
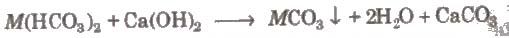
Removal of Permanent Hardness
(i) By adding washing soda The calcium or magnesium salts are precipitated as carbonates.

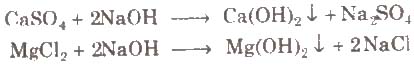
(ii) By adding caustic soda The temporary and permanent hardness can be removed by adding caustic soda.
(iii) By adding sodium phosphate(Na3PO4) The phosphates of calcium and magnesium are precipitated.
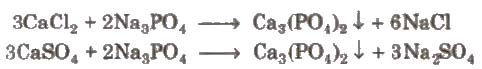
Similarly, magnesium also precipitate out in the form of magnesium phosphate, Mg3(PO4)2.
(iv) Calgon’s process Calgon is sodium hexa metaphosphate (Na6P6O18). This calgon when added to hard water form soluble complex.

Similarly. Mg2+ can also precipitate as Na2[Mg2(PO3)6] and water becomes free from Ca2+ and Mg2+ Ions.
(v) Permutit process Permutit is hydrated sodium aluminium silicate Na2Al2Si2O8.xH2O. It exchanges its sodium ions for divalent ions Ca2+ and Mg2+..
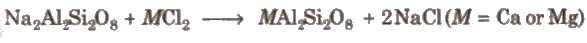
Permutit when fully exhausted can be regenerated by treating with 10% solution of sodium chloride. It is most efficient method to gel water with zero degree of hardness.
(vi) By synthetic resins
These are of two types:
(a) Cation exchange resins are big molecules containing sulphonic acid group (-SO3H). It is first changed into sodium salt with the general formula RNa. The hard water is passed through it so Ca2+and M2+ are exchanged and removed.
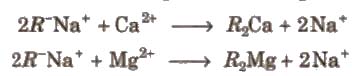
The resins like permutit can be regenerated with a solution of NaCl.
(b) Anion exchange resins are also big molecules and can exchange anions. They contain an amino group.
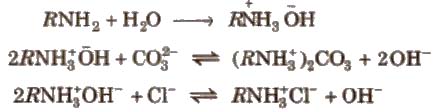
The water is first passed through cation resins and then through anion tesin and pure distilled water is obtained.
Measurement of Degree of Hardness
Degree of hardness is defined as the number of parts of calcium carbonate or equivalent to various calcium and magnesium salts present in one million parts of water by mass. It is expressed in ppm.
Degree of hardness (in ppm) = (wt. of CaCO3 (g)/ wt. of hard water (g)) x 106
The molecular wt. of Ca(HCO3)2, Mg(HCO3)2, CaCl2, MgCl2, CaSO4 and MgSO4 is 162, 146, 111, 95, 136 and 120 respectively. The mol. wt. of CaCO3 is 100.
Thus, 162 g Ca(HCO3)2, 146 g Mg(HCO3)2, 111 gCaCl2, 95 g MgCl2136 g CaSO4 and 120 g MgSO4 are equivalent to 100 g CaCO3.
Hydrogen Peroxide [H2O2]
H2O2 was discovered by J.L. Thenard in 1818. It is an important compound used in pollution control treatment of domestic and industrial effluents.
Methods of Preparation
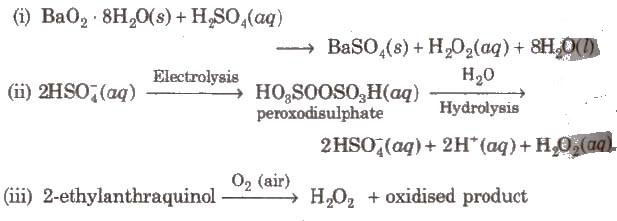
Strength of Hydrogen Peroxide
The most common method to express the strength of H2O2 is in terms of the volume (in mL) of oxygen liberated at NTP by decomposition or 1 mL of that sample of H Z 0 2. A solution of H2O2 labelled as ’10 volume’ actually means “1 mL of such a solution of H2O2 on decomposition by heat produces 10 mL of oxygen at NTP”.
(i) Strength of H2O2 in terms of normality
(68 x X/22.4) = 17 x N ⇒ X = 5.6 x N
where, X is volume strength of H2O2.
(ii) % strength = (17/56) x volume strength
(iii) X = 11.2 x molarity.
Storage of Hydrogen Peroxide (H2O2)
It is stored in the presence of traces of alcohol, acetanilide or sodium pyrophosphate which slow down the rate of decomposition of hydrogen peroxide.
Chemical Properties of H2O2
- Acidic nature It is weakly acidic in nature and pure hydrogen peroxide turns blue litmus red.
- Oxidising agent It acts as a strong oxidising agent in acidic as well as in basic medium.
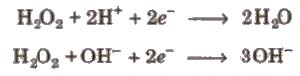
e.g., oxidising action of HzOz is
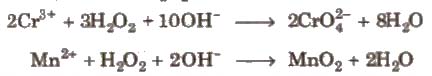
(iii) Reducing agent
(a) In acidic medium
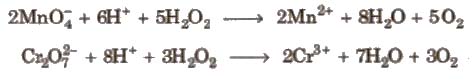
(b) In basic medium
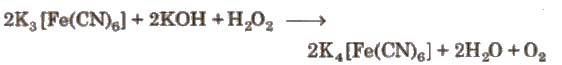
(iv) Bleaching properties Its bleaching action is due to oxidation by atomic oxygen and permanent.
H2O2 rarr; H2O + [O]
dye + [O] → dye is oxidised and bleached

