Class 11 Chemistry Chapter P-Block elements Notes

Group 13
It is also called boron family. It includes B, Al, Ga, In, Tl. AI is the most abundant metal and third most abundant element in the earth’s crust.
General Physical Properties of Group 13 Elements
(i) Electronic configuration Their valence shell electronic configuration is ns2np1
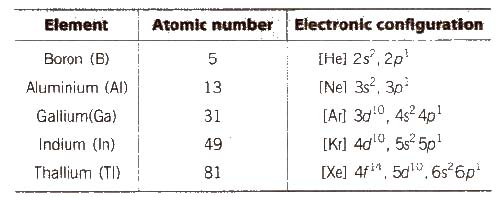
(ii) Atomic radii and ionic radiiGroup 13 elements have smaller size than those of alkaline earth metals due to greater effective nuclear charge, Zeff’
Atomic radii increases on going down the group with an anomaly at gallium (Ga). Unexpected decrease in the atomic size of Ga is due to the presence of electrons in d-orbitals which do not screen the attraction of nucleus effectively.
The ionic radii regularly increases from B3+ to TI3+.
(iii) Density It increases regularly on moving down the group from B to Tl.
(iv) Melting and boiling pointsMelting point and boiling point of group 13 elements are much higher than those of group 2 elements. The melting point decreases from B to Ga and then increases, due to structural changes in the elements.
Boron has a very high melting point because of its three dimensional structure in which B atoms are held together by strong covalent bonds.
Low melting point of Ga is due to the fact that it consists of Ga2molecules, and Ga remains liquid upto 2276 K. Hence, it is used in
high temperature thermometer.
high temperature thermometer.
(v) Ionisation enthalpy (IE) The first ionisation enthalpy values of group 13 elements are lower than the corresponding alkaline earth metals, due to the fact that removal of electron is easy. [ns2 npl configuration] .
On moving down the group, IE decreases from B to Al, but the next element Ga has slightly higher ionisation enthalpy than A1 due to the poor shielding of intervening d-electrons. It again decreases in In and then increases in the last element Tl
(vi) Oxidation states B and Al show an oxidation state of +3 only while Ga, In and TJ exhibit oxidation states of both +1 and +3.
As we move down in the group 13. due to inert pair effect, the tendency to exhibit +3 oxidation state decreases and the tendency to attain +1 oxidation state increases.
Stability of +1 oxidation state follows the order Ga < In < Tl.
Inert pair effect is reluctance of the s-electrons of the valence shell to take part in bonding. It occurs due to poor shielding of the ns2 – electrons by the intervening d and f – electrons. It increases down the group and thus, the lower elements of the group exhibit lower oxidation states.
(vii) Electropositive (metallic) character These elements are less electropositive than the alkaline earth metals due to their smaller size and higher ionisation enthalpies.
On moving down the group, the electropositive character first increases from B to Al and then decreases from Ga to TI, due to the presence of d and I-orbitals which causes poor shielding.
(viii) Reducing character It decreases down the group from AI to Tl because of the increase in electrode potential value for M3+ / M.
Therefore, it follows the order
AI> Ga > In > Tl
(ix) Complex formation Due to their smaller size and greater charge, these elements have greater tendency to form complexes than the s-block elements.
(x) Nature of compounds The tendency of the formation of ionic compounds increases from B to Tl. Boron forms only covalent compounds whereas AI can form both covalent as well as ionic compounds. Gallium forms mainly ionic compounds, although anhydrous Ga CI3 is covalent.
Chemical Properties of 13 Group Elements
(i) Action of air Crystalline boron is unreactive whereas amorphous boron is reactive. It reacts with air at 700°C as follows
4B + 3O2 → 2B2O3
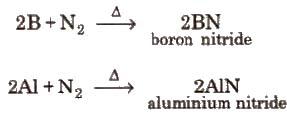
2B + N2 → 2BN
AI is stable in air due to the formation of protective oxide film.
4Al + 3O2 → 2Al2O3
Thallium is more reactive than Ga and In due to the formation of unipositive ion, TI+.
4Tl + O2 → 2Tl20
(ii) Reaction with nitrogen
(iii) Action of water Both B and AI do: not react with water but amalgamated aluminium react with H2O evolving H2.
2Al(Hg) + 6H2O )→ 2AI(OH)3 + 3H2 + 2Hg
Ga and In do not react with pure cold or hot water but Tl forms an oxide layer on the surface.
(iv) Reaction with alkalies Boron dissolves in alkalies and gives sodium borates.
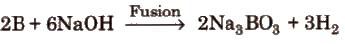
Aluminium also reacts with alkali and liberates hydrogen.
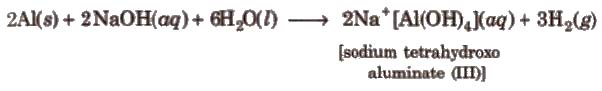
(v) Reaction with carbon
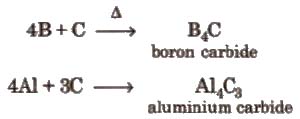
Aluminium carbide is ionic and forms methane with water.
(vi) Hydrides Elements of group 13 do not combine directly with H2 to form hydrides, therefore their hydrides have been prepared by indirect methods, e.g
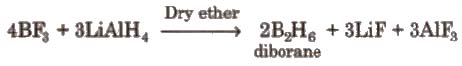
Boron forms a number of hydrides, they are known as boranes. Boranes catch fire in the presence of oxygen.
B2H6 + 3O2 → B2O3 + 3H2O; &Delat;cH° = – 1976 kJ mol-l
Boranes are hydrolysed by water.
B2H6 + 6H2O → 2H3BO3 + 6H2
Boranes are stable but the stability of hydrides of AI, Ga, In, and Tl decreases on moving down the group because the strength of the M-H bond decreases.
Structure of diborane BH3 does not exist as such, but exists as a dimer, i.e; B2H6(diborane].
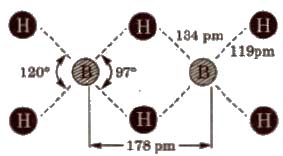
In the above structure, B atoms are in Sp3 – hybrid state. There are six B-H bonds out of which four B-H bonds are normal bonds present in the same plane While rest two B-H bonds behave as bridge bonds, ie; 3c – 2e (three centre-two electrons, also known as banana bond) and present above and below the plane of the molecules which do not I have sufficient number of electrons to form covalent bonds.
Aluminium (AI) forms a polymeric hydride of general formula (AIH3)xwhich decomposes into its elements on heating.
(vii) Oxides Except. TI all the elements of group 13 form oxides or general formula M2O3 on heating with oxygen.
TI forms thallium (l) oxide. Tl2O which IS more stable than thallium (III) oxide TI2O3 due to inert pair effect.
(viii) Nature of oxides and hydroxides B(OH)3 or H3BO3 is soluble in water, while other hydroxides are insoluble in water.
On moving down the group. there is a change from acidic to amphoteric and then to basic character of oxides and hydroxides or group 13 elements.
(ix) Halides All the elements of boron family (except Tl) form trihalides of type MX3.
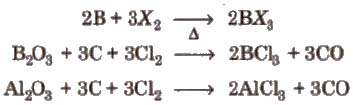
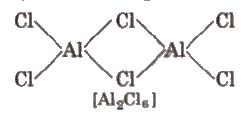
All the boron trihalides [BX3) and aluminium trihalides AlX3 (except AIF3 which is ionic) are covalent compounds. AlX3 exists as dimer while BX3 is monomer because boron atom is too small to coordinate with four large halide ions. The energy released during the formation of the bridge structure is not sufficient for the cleavage of the typical pπ – pπ bond in BF3.
BF3 is a colourless gas, BCl3 and BBr3 are colourless fuming liquids and BI3 is a white solid at room temperature.
Trihalides of group 13 elements behave as Lewis acids because of their strong tendency to accept a pair of electrons. The relative strength of Lewis acids of boron trihalides is
BF3 < BCI3, < BBr3, < BI3.
This is due to pπ – pπ backbonding in BF3 which makes it less electron deficient.
The halides of group 13 elements behave as Lewis acids and the acidic character is
BX3 > AIX3 > GaX3 > InX3 (where, X = Cl, Br or 1)
TICI3 decomposes to TICl and C12and hence acts as an oxidising agent.
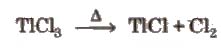
Anomalous Behaviour of Boron
Boron shows anomalous behaviour with the other members of the group, due to the following reasons:
(i) Smallest size in the group.
(ii) High ionisation energy.
(iii) Highest electronegativity in the group.
(iv) Absence of vacant d-orbital.
(ii) High ionisation energy.
(iii) Highest electronegativity in the group.
(iv) Absence of vacant d-orbital.
A few points of difference are
1. It is a non-metal while other members of the group are metallic.
2. It shows allotropy while other members do not.
3. It has the highest melting point and boiling point in group 13.
4. It forms only covalent compounds while other members form both ionic and covalent compounds.
5. The halides of boron exist as monomers while AlCI:! exists as a dimer.
6, The oxides and hydroxides of boron are weakly acidic while those of aluminium arc amphoteric and those of other elements are basic.
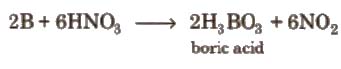
7. It can be oxidised by concentrated HNO3 while aluminium becomes passive due to the formation of oxide layer on the surface.
2. It shows allotropy while other members do not.
3. It has the highest melting point and boiling point in group 13.
4. It forms only covalent compounds while other members form both ionic and covalent compounds.
5. The halides of boron exist as monomers while AlCI:! exists as a dimer.
6, The oxides and hydroxides of boron are weakly acidic while those of aluminium arc amphoteric and those of other elements are basic.
7. It can be oxidised by concentrated HNO3 while aluminium becomes passive due to the formation of oxide layer on the surface.
Diagonal Relationship between Boron and Silicon
Boron exhibit resemblance with its diagonal element silicon of group 14.
1. Both Band Si are non-metals.
2. Both arc semi-conductors.
3. Both Band Si form covalent hydrides, i.e.. boranes and silanes respectively.
4. Both form covalent, and volatile halides which fume in moist air due to release of HCI gas.
2. Both arc semi-conductors.
3. Both Band Si form covalent hydrides, i.e.. boranes and silanes respectively.
4. Both form covalent, and volatile halides which fume in moist air due to release of HCI gas.
BCI3 + 3H2O → H3 BO3 + 3HCl i
SiCl4 + 4H2O → Si(OH)4 + 4HCl
5. Both form solid oxides which get dissolve in alkalies forming borates and silicates respectively. ..
6. Both react with electropositive metals and give binary compounds, which yield mixture of boranes and silanes on hydrolysis.
6. Both react with electropositive metals and give binary compounds, which yield mixture of boranes and silanes on hydrolysis.
Boron and Its Compounds
Occurrence
It does not occur in free state. Its important minerals are
(i) Borax (or Tineal), Na2B4O7 * 1OH2O
(ii) Kernite, Na2B4O7 * 4H2O
(iii) Orthoboric acid, H3BO3
(ii) Kernite, Na2B4O7 * 4H2O
(iii) Orthoboric acid, H3BO3
Isolation
Elemental boron is obtained by following methods :
(i) By reduction of boric oxide with highly electropositive metals like K, Mg, AI, Na etc, in the absence of air.
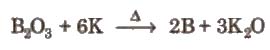
(ii) By the reaction of boron halides with hydrogen,
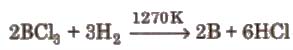
Uses of Boron
(i) As a semi-conductor.
(ii) Boron steel rods are used to control the nuclear reactions.
(ii) Boron steel rods are used to control the nuclear reactions.
5B10 + 0n1 → 5B11
1. Borax or Sodium Tetraborate Decahydrate [Na2B4O7 * 1OH2O]
Preparation
It occurs naturally as tineal in dried up lakes. It is obtained by boiling of mineral colemanite with a solution of Na2Co3.
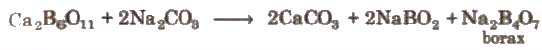
NaBO2 can be removed by passing CO2 through it.
4NaBO2 + CO2 → Na2CO3 + Na2B<4O7
Properties
1. Its aqueous solution is basic in nature.
Na2B4O7 + 7H2O → 2NaOH + 4H3BO3
2. On heating with ethyl alcohol and cone.H2SO4it gives volatile vapours of triethyl borate which burn with a green flame.
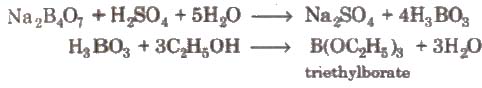
3. Action of heat
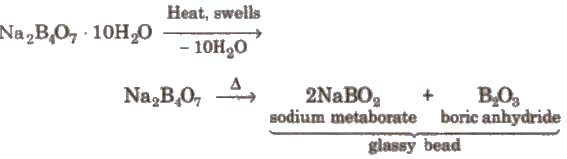
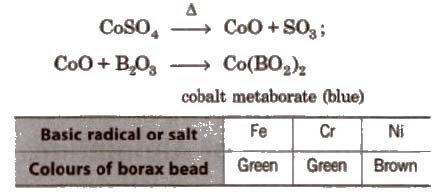
Borax bead is used for the detection of coloured basic radicals under the name borax bead test e.g.,
2. Boric Acid or Orthoboric Acid[H3BO3 or B(OH)3]
Preparation
By treating borax with dil. HCl or dil. H2SO4.
Na2B4O7 + 2HCl + 5H2O → 2NaCI + 4H3BO3
Properties
1. It is a weak monobasic acid (Lewis acid).
H3BO3 + 2H2O → [B(OH)4]– + H3O+
2. With C2H5OH and cone H2SO4, it gives triethyl borate.
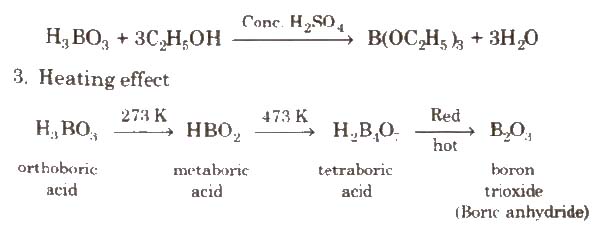
Uses
It is used as an antiseptic and eye lotion under the name ‘boric lotion’, and as a food preservative.
3. Borazine or Borazole, [B3N3H6]
It is a colourless liquid having a six membered ring of alternating B and N atoms. It is also called ‘inorganic benzene’. It is prepared by B2H6 as follows
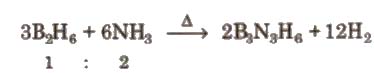
The π electrons in borazine are only partially delocalised. It is more reactive than benzene
Compounds of Aluminium
1.Anhydrous Aluminium Chloride[AICI3 or A12C16]
Preparation
It can not be prepared by heating AICI3. 6H2O.
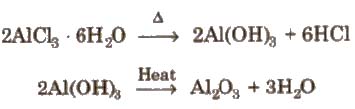
It can be prepared
(i) By passing dry chlorine or HCl gas over heated Al.
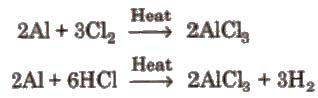
(ii) By heating a mixture of alumina and carbon in a current of dry chlorine.
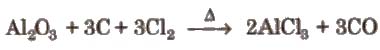
Properties
1. AlC13 fumes in moist air due to hydrolysis.
AlC13 + 3H2O → Al(OH)3 + 3HCI
2. It behaves as Lewis acid.
Uses
It is used as a catalyst in Friedel-Craft reaction and as a mordant dye.
2.Aluminium Oxide or Alumina[AI2O3]
It is the most stable compound of aluminium and occurs in nature as colourless corundum and several coloured oxides, (it present in combination with different metal oxides) like ruby (red), topaz (yellow), sapphire (blue), and emerald (green), which are used as precious stones (gems).
Alum
The term alum is given to double sulphates of the type X2SO4 * Y2(SO4)3 * 24H2O where, X represents a monovalent cation such as Na+, K+ and NH+4, while Y is a trivalent cation such a Al3,Cr3+, Fe3+ and Co3+(Li+ does not form alum).
Some important alums are
(i) Potash alum K2SO4 * Al2(SO4)3 * 24H2O
(ii) Sodium alum Na2SO4 * A12(SO4)3. 24H2O
(iii) Ammonium alum (NH4)2SO4 * AI2(SO4)3 24H2O
(iv) Ferric alum (NH4)2SO4 * Fe2(SO4)3 24H2O
Potash alum is prepared in the laboratory by mixing hot equimolar quantities of K2SO4 and Al2(SO4)3. The resulting solution on concentration and crystallisation gives potash alum.
Note 1. A mixture of Al powder NH4NO3 is called ammonal and is lUed in bombs.
2. Al is the chief constituent of silver paints.
3. A12(SO4)3 1.8 used for making fire proof clothes.
Group 14
General Physical Properties of Group 14 Elements
(i) Electronic configuration Their valence shell electronic configuration is ns2 np2
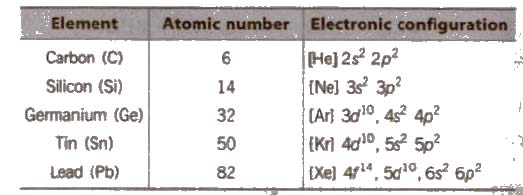
(ii) Metallic character C and Si are non-metals, Ge is a metalloid and Sn and Pb are metals.
(iii) Appearance C is black. Si is light-brown, Ge is greyish, Sn and Pb are silvery white.
(iv) Density Density increases with increase m atomic number due to increase in mass per unit volume down the group.
(v) Melting points and boiling points The melting points and boiling points decrease from carbon to lead but carbon and silicon have very high melting and boiling points due to their giant structure.
(vi) Oxidation state They exhibit +2 and +4 oxidation state. The compounds of Pb in +4 oxidation state are powerful oxidising agents since, +2 oxidation state of Pb is more stable due to inert pair effect.
The compounds in +2 oxidation state are ionic in nature and in + 4 oxidation state are covalent in nature (According to Fajan’s rule).
(vii) Ionisation enthalpy It decreases from C to So. For Pb. it ie slightly higher than Sn.
(viii) Electronegativity values The value decreases from C to Pb but not in a regular manner probably due to filling of d-orbitals III and Sn and f- orbitals In Pb.
(ix) Catenation The greater the strength of element-element bond. the greater is the strength of catenation.
C >> Si > Ge = Sn > Pb (catenation).
(x) Allotropy All the elements of this group except Pb exhibit allotropy.
In cold countries white tin changes to grey tin and results in decrease in density. This is called tin disease or tin plague.
(xi) Valency All elements exhibit tetra valency. In case of carbon, 406 kJ mol-1 of energy is required for promotion of 2s – electron to 2p.
Formation of two extra bonds provide this energy.
(xii) Atomic and ionic radii Both increase from C to Pb.
(xiii) Multiple bonding Carbon forms pπ – pπ bonds with itself and with S, N and O. Other clements show negligible tendency of this
type due to their large size. Others form dπ – pπ multiple bonds.
type due to their large size. Others form dπ – pπ multiple bonds.
Chemical Properties of Group 14 Elements
(a) Hydrides All members of the group form covalent hydrides. Their number and ease of formation decreases down the group.
Hydrides of carbon are called hydrocarbons (alkanes, alkenes or alkynes).
Hydrides of Si and Ge are known as silanes and germanes.
The only hydrides of Sn and Pb are SnH4 (stannane) and PbH4(plumbane),
Their thermal stability decrease down the group.
Their reducing character increases down the group.
(ii) Halides All the elements give tetrahedral and covalent halides of the type MX4 except PbBr4, and PbI4.
Thermal stability
CX4 > SiX4 > GeX4 > SnX4 > PbX4
Order of thermal stability with common metals
MF4 > MCl4 > MBr4 > MI4
Except CX4 other tetrahalides can hydrolysed due to the presence of vacant d-orbitals.
SiX4 + 2H2O → SiO2 + 4Hx.
ease of hydrolysis: SiX4 > GeX4 > SnX4 > PbX4
Except C, other elements form dihalides of the type MX2 which are nlOre ionic and have higher melting points and boiling points, e.g., SnC12 is a solid whereas SnC14is a liquid at room temperature.
SnCl2 . 5H2O is called bitter of tin and is used as a mordant in dyeing.
(iii) Oxides They form two types of oxides. mono-oxides of the type MO. e.g.,
CO (neutral) and SiO, GeO. SnO. PbO(all basic) and dioxides of the type MO2
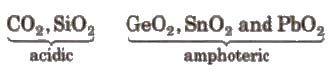
CO2 is linear gas at ordinary temperature. Solid CO2 is known as dry ice or drikold.
SiO2 is a solid with three dimensional network in which Si is bonded to four oxygen atoms tetrahedrally and covalently. A mass of hydrated silica (SiO2) formed from skeletons of minute plants, known as diatoms, is called kieselguhr. It is a highly parous material and is used in the manufacture of dynamite.
Carbon
Free states (diamond. graphite, coal etc.) and combined states (oxides, carbonates, hydrocarbons etc.)
Allotropic Forms of Carbon
The crystalline forms include
(i) Diamond It is the hardest and has three dimensional polymeric structure in which hybridisation of C is sp3. It is covalent solid. melting point 3650°C. density 3.51 g/cm3and bad conductor of heat and electricity.
(ii) Graphite It is dark grey. having hexagonal plates, hybridisation of each C is sp2. It is good conductor of heat and electricity due to the presence of free electrons. It was also known as black lead. It is a very good lubricant.
Aqua dag Suspensions of graphite in water.
Oil dag Suspension of graphite in oil lubricants.
(iii) Fullerenes These are the only pure form of carbon. C60 molecule contains 12 five membered rings and 20 six membered rings. The five membered rings are connected to six membered rings while six membered rings are connected to both five and six membered rings. These are used in microscopic ball bearings, light weight batteries, in synthesis of new plastics and new drugs.
Amorphous forms of carbon are
(i) Coal The different forms of coal are peat (60 % C), lignite (70 % C), Bituminous (78 % C), Semi Bituminous (83 % C) and anthracite (90 % C). Bituminous is most common variety of coal.
(ii) Coke It is obtained by destructive distillation of coal
(iii) Charcoal or wood charcoal It is obtained by heating wood strongly in absence of air. When heated with steam, it becomes more activated. It is used to remove colouring matters and odoriferous
gases.
gases.
(iv) Bone black or animal charcoal It is obtained by destructive distillation of bones in iron retort. By products are bone oil or pyridine. It is used as adsorbent. On burning, it gives bone ash which is calcium phosphate and used in the manufacture of phosphorous and phosphoric acid.
(v) Lamp-black It is obtained by burning vegetable oils in limited supply of air. It is used in the manufacture of printing ink, black paint, varnish and carbon paper.
(vi) Carbon-black It is obtained by burning natural gas in limited supply of air. It is added to rubber mixture for making automobile tyres.
Coal Gas
Preparation By destructive distillation of coal.
Composition
H2 = 45 – 55 % N2 = 2 – 12 %
CH4 = 25 – 35 % CO2 = 0 – 3 %
CO = 4 – 11 % O2 = 1 – 1.5 %
CH4 = 25 – 35 % CO2 = 0 – 3 %
CO = 4 – 11 % O2 = 1 – 1.5 %
Ethylene, acetylene, benzene, etc. = 3 – 5 %
Uses It is used as illuminant, as fuel and to provide inert atmosphere in the metallurgical processes.
Natural Gas
It is found along with petroleum below the surface of earth.
Composition CH4 = 60 – 80 %
Higher hydrocarbons = 2 – 12%
C2H6 = 5 – 10 %, C3H8 = 3 – 18 %
Uses It is used as a fuel.Its partial combustion yields carbon black (reinforcing agent for rubber).
Oil Gas
Preparation

Uses It is used as fuel in laboratories in Bunsen burners.
Wood Gas
Preparation Destructive distillation of wood gives wood gas (CH4, C2H6 H2)
Uses It is used as fuel.
Liquified Petroleum Gas (LPG)
Composition n-butane + Iso-butane
Uses It is used as domestic fuel.
Carbon Monoxide (CO)
Preparation
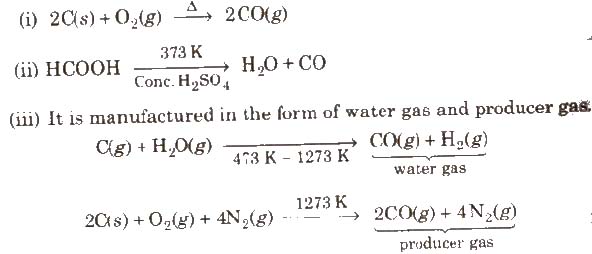
Properties
It is colourless, odourless and almost water insoluble gas. It is a powerful reducing agent. CO is used in the extraction of many metals from their oxide ores.
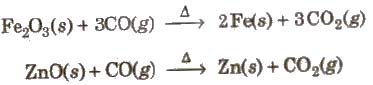
Carbon Dioxide (CO2)
Preparation
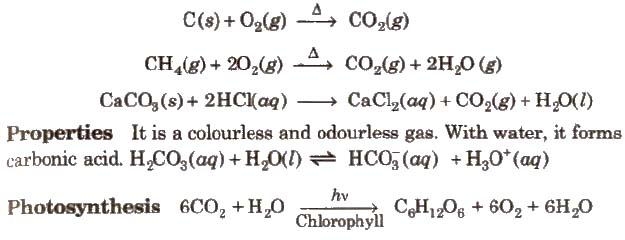
Compounds of Silicon
Silicates
Silicates are metal derivatives of silicic acid, H2SiO3 and can be obtained by fusing metal oxides or metal carbonates with sand. The basic structural unit of silicates is SiO– 44.
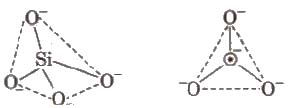
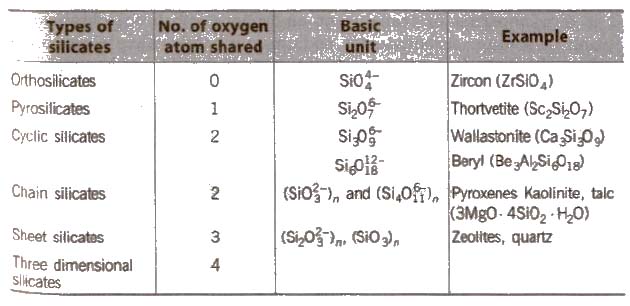
Talc consists of planar sheets which can slip over one another due to weak forces of attraction, and is a constituent of talcum powder. That’s
Why talcum powder has a slippery touch.
Why talcum powder has a slippery touch.
Mica (abrak) is naturally occurring aluminium silicate [KH2AI3(SiO4]3 or KAI3Si3O10(OH)2.
Silicones
The linear, cyclic or cross linked polymeric compounds containing (R2SiO) as a repeating units, are known as silicones. They are manufactured from alkyl substituted chlorosilanes.
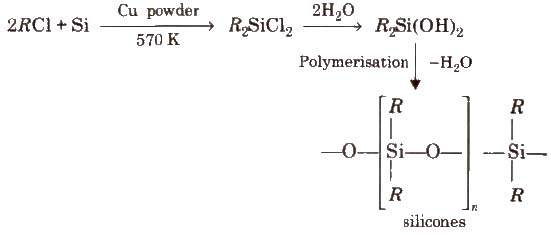
Silicones are chemically inert, water repellent, heat resistant, good electrical insulators. These are used as lubricants (vaseline), insulators etc.
Carborundum
It is second hardest material known and has formula SiC (silicon carbide), It is used as high temperature semiconductor, in transistor diode rectifiers.
Glass
it is a transparent or translucent amorphous substance obtained by fusion of sodium carbonate (or sodium SUlphate), calcium carbonate and sand (silica). It is not a true solid, so its melting point is not sharp.
General formula of glass is Na2O * CaO * 6SiO2.
Coloured glasses are obtained by adding certain substance to the molten mass.
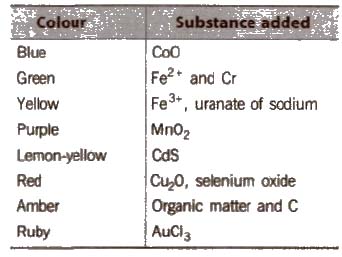
Different Varieties of Glass
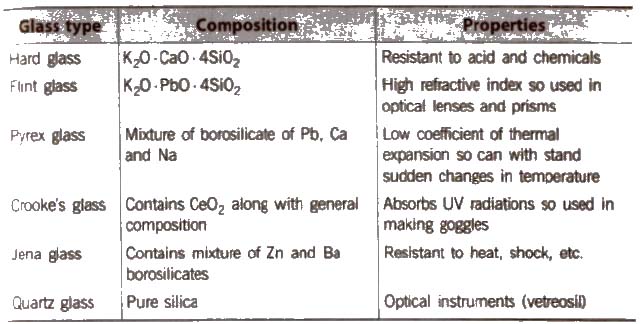
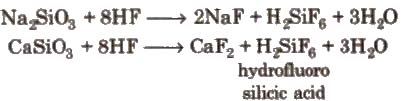
Glass is attacked by HF. This property is used in the etching of glass
Compounds of Lead
Chrome yellow (PbCrO 4 )
It is prepared by adding potassium chromate to lead chromate and is used as a yellow pigment under the name chrome yellow. On treating with alkali. it gives basic lead chromate or chrome red, PbCrO4 * PbO.
Basic lead carbonate, Pb(OH)2 . 2PbCO3
It is also known as white lead and is prepared by adding sodium carbonate solution to any lead salt.
3Pb(NO3)2 + 3Na2CO3 + H2O → Pb(OH)2 * 2PbCO3 + 6NaNO3 + CO2
It is used as white paint. The disadvantage of using white lead in paints is that it turns black by the action of H2S of the atmosphere.
lead poisoning is called plumbosolvency which increases in the excess of nitrates, organic acids and ammonium salts.
Group 15
The 15 group of the Periodic Table consists of nitrogen. phosphorus. arsenic, antimony and bismuth. These elements are known as pnicogens and their compounds as pniconides.
Physical Properties of Group 15 Elements
(i) Electronic configuration Their valence shell electronic configuration is ns2 np3
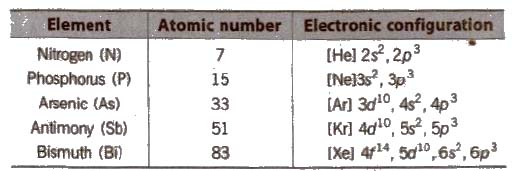
(ii) Metallic character N and P are non-metals, As and Sb are metalloids and Bi is metal.
(iii) Physical state Nitrogen is the first element after hydrogen which is diatomic gas in native form. All other elements in the group are solids.
(iv) Atomicity N2 is diatomic while others are triatomic E4.
(V) Melting and boiling points The melting point increases from nitrogen to arsenic. The boiling points increase regularly on moving down the group.
(Vi) Density It increases down the group.
(Vii) Atomic radii It increases with increase in atomic number as we go down the group.
(viii) Allotropy All the elements (except Bi) exhibit allotropy. Nitrogens – α nitrogen, β – nitrogen.
(viii) Allotropy All the elements (except Bi) exhibit allotropy. Nitrogens – α nitrogen, β – nitrogen.
Phosphorus – White, red, black
Arsenic – Grey, yellow, black
Antimony – Metallic yellow (explosive)
Arsenic – Grey, yellow, black
Antimony – Metallic yellow (explosive)
(ix) Oxidation state
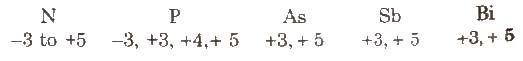
Nitrogen has a wide range of oxidation states.
The stability of +3 oxidation state increases and stability of +5 oxidation state decreases on moving down the group due to inert pair effect.
(x) Ionisation enthalpy Ionisation energy of nitrogen is very high due to its small size and half-filled highly stable configuration. The ionisation energy decreases down the group.
(xi) Electronegativity It decreases from nitrogen to bismuth.
(xii) Catenation ‘They exhibit the property of catenation but to lesser extent due to weak E – E bond than 14 group elements.
(xiii) Reactivity Elemental nitrogen is highly unreactive because of its strong triple bond. (almost as inert as noble gases).
White phosphorus is extremely reactive and kept in water. It is inflammable and can be ignited at 45°C.
Chemical Properties of Group 15 Elements
(i) Hydrides All the elements of this group form hydrides of the type EH3, which are covalent and pyramidal in shape. Some properties follows the order as mentioned
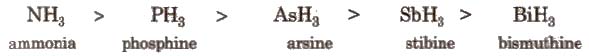
[These properties are
1. Thermal stability,
2. Basic strength,
3. Solubility in water,
4. Bond angle NH3 (107.4°); PH3(92°),AsH3 (91° ), SbH3(90° ),
5. Strength of M – H bond
2. Basic strength,
3. Solubility in water,
4. Bond angle NH3 (107.4°); PH3(92°),AsH3 (91° ), SbH3(90° ),
5. Strength of M – H bond
Some properties follow the order
NH3 < PH3 < AsH3 < SbH3 < BiH3
[These properties are
1. Reducing character
2. Covalent character
3. Rate of combustion
2. Covalent character
3. Rate of combustion
(ii) Halides All the elements of this group form trihalides, MX3 and except nitrogen all form pentahalides, MX5, e.g., NCi3, NI3, PCI3, BiCI3, AsCI3 , PCl5 etc. Trihalides (except of N) behaves as Lewis acid and the order of their strength is PCl3 > AsCl3 > SbCl3Trihalides of N behave as Lewis base and has the following order of strength
NF3 < NCl3 < NBr3 < NI3
NCl3 is an explosive compound
(iii) Oxides All the elements of this group form oxides of the type M2O3and M2O5.
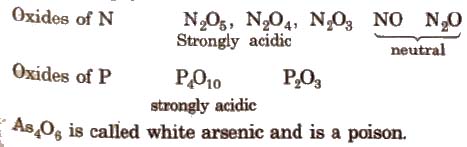
The acidic strength of pentoxide and trioxides decrease on moving down the group, i.e.,
N2O5 > P2O5 > As2O5 > Sb2O5
BiOCI is called pearl white.
Nitrogen and its Compounds
1. Dinitrogen (N2)
Preparation
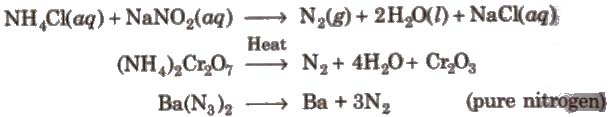
Properties
1. Nitrogen does not react with alkali metals except Li but reacts with alkaline earth metals to give metal nitride.
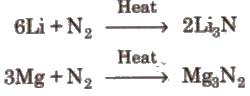
2. Reaction with oxygen
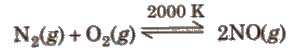
3. Reaction with non-metals
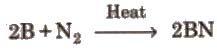
4. Reaction with CaC2
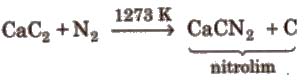
Uses Liquid N2 is used as refrigerant. Nz is used in the manufacture of HNO2, NH2, CaCN2(calcium cyanamide) and other nitrogenous compounds. It is used for filling electric bulbs.
2. Ammonia (NH3)
Preparation
(i) Lab method
2NH4Cl + Ca(OH)2 → CaCI2 + 2NH3 + 2H2O
(ii) Haber’s process
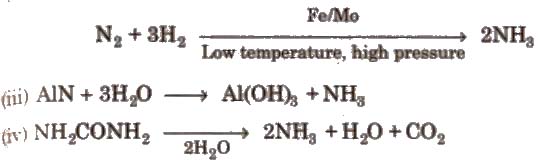
Properties
1. It is a colourless gas with characteristic pungent odour. It is extremely soluble in water due to H – bonding.
2. It is a strong Lewis base and used in the metal ion detection as
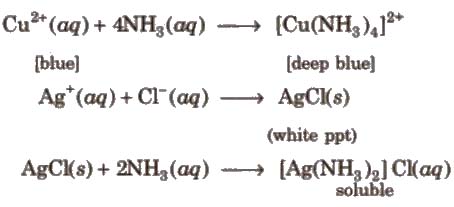
3. Reaction with chlorine
When NH3 is in excess, N2 is the main product.
8NH3 + 3C12 → 6NH4CI + N3
When C12 is in excess, NCl3 is the main product.
NH3 + 3C12 → NCl3 + 3HCl
4. Reaction with Nesseler’s reagent
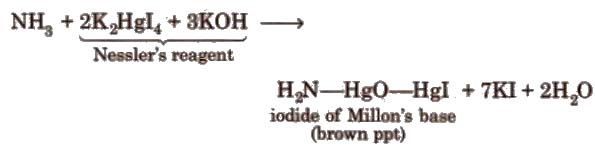
Uses It is used as a refrigerant and to produce various nitrogenous fertilizers.
Oxides of Nitrogen
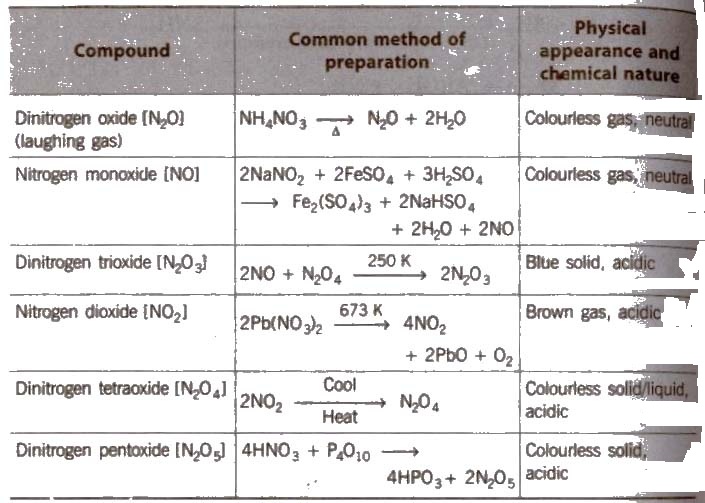
NO2 contains odd number of valence electrons. On dimerisation. it is converted to stable N2O4molecule with even number of electrons .
3.Nitric acid (HNO3)
It is a stronger acid than H3PO4.
Preparations
(i) Lab method
NaNO3 + H2SO4 (cone.) → NaHSO4 + HNO3
(ii) Ostwald’s process
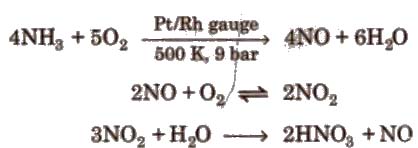
Physical properties It is a syrupy, colourless, pungent liquid usually available as 68 % and 15.7 M aqueous solution is often yellow due to small concentrations of NO2.
Chemical reactions
1. Action of nitric acid on zinc under different conditions
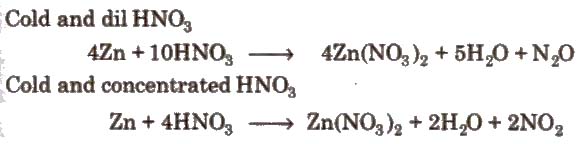
2. Action of nitric acid on copper under different conditions
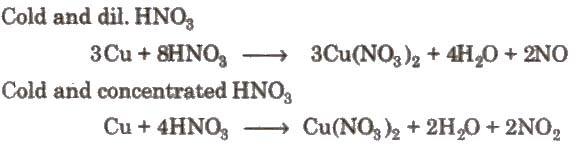
3. Reaction with non-metals
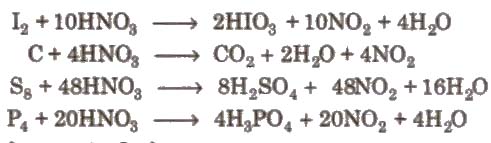
4. Brown ring test of nitrate
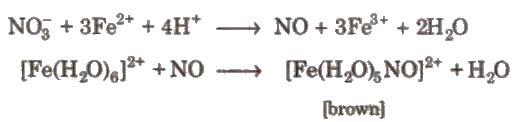
5. Metals like Fe. Cr. Ni, AI or Co becomes inactive or passive due to stable oxide layers.
Structure of nitric acid
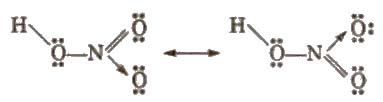
Uses It is used
1. in the manufacturing of fertilizers.
2. for purification of silver and gold.
3. in the manufacturing of explosives and as oxidising agent.
4. as nitrating reagent
2. for purification of silver and gold.
3. in the manufacturing of explosives and as oxidising agent.
4. as nitrating reagent
Phosphorus and its Compounds Allotropic Forms of Phosphorus
(i) White phosphorus
(ii) Red phosphorus
(iii) Black phosphorus
(ii) Red phosphorus
(iii) Black phosphorus
Some Points of Distinction Between White and Red Phosphorus
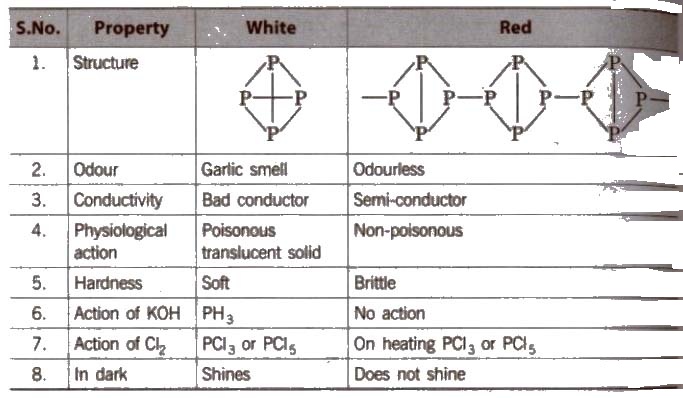
Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803 K. It does not oxidise in air.
Match box side contains red P or P2S3 + glue and on tip of match stick. red P, KelO3 chalk and glue is deposited.
Chemical properties
1. With non-metals
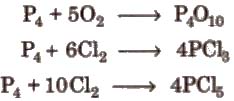
2. With compounds
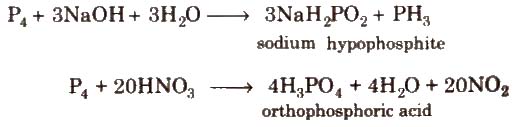
Uses It is used in match boxes, explosives, as rat poison, in fertilizers and alloys
1. Phosphine (PH3)
Preparation It is prepared by following methods
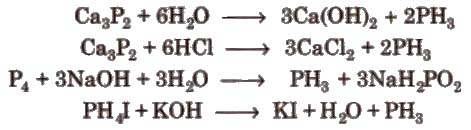
Properties
1. It is a colourless gas with rotten fish like smell and is highly poisonous. It explodes in contact. with traces of oxidising agents like HNO3,C12 and Br2 vapours.
3CuSO4 + 2PH3 → CU3P2 + 3H2SO4
3HgCl2 + 2PH3 → Hg3P2 + 6HCI
2. Phosphine is weakly basic.
PH3 + HBr → PH+4Br–
Uses It is used to prepare smoke screens in warfare. A mixture of CaC2 and Ca3P2 is used in Holme’s signals.
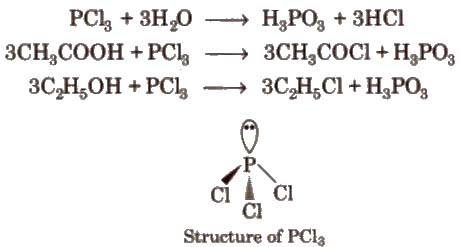
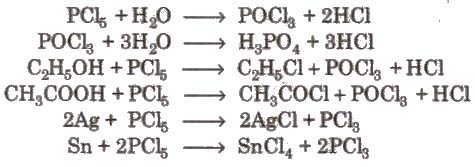
2. Phosphorus Trichloride (PCl5)
Preparation

Properties It is a colourless oily liquid. having pyramidal shape [sp3– hybridised]
3.Phosphorus Pentachloride(PCI5)
Preparation
P4 + 10 Cl2 → 4 PCl5
P4 + 10 SO2CI2 → 4PCl5 + 10 SO2
Structure PCl5 in gaseous and liquid phases has sp3d – hybridization and its shape is trigonal bipyramidal. The three equatorial P – CI bonds are equivalent while the two axial bonds are longer equatorial bonds.
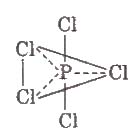
Properties In solid state, PCI5exists as an ionic solid, [PCI4]+[PCl6]– in which, the cation, [PCI4]+ is tetrahedral and the anion [PCl6]– is octahedral.
Oxoacids of Phosphorus
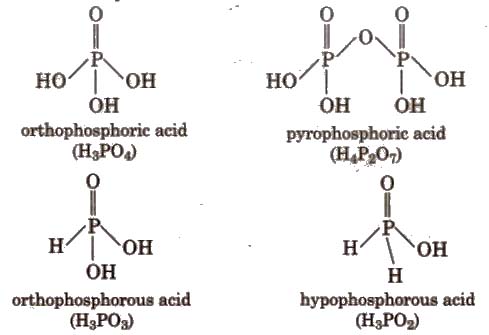
In toothpaste, CaHPO4 * 2H2O is added as mild abrasive and polish agent.
Group 16
The elements oxygen (0), sulphur (S), selenium (Se), tellurium (Te) and polonium (Po) belong to group 16 of the Periodic Table. These elements are known as chalco gens, i.e., ore forming elements.
The name sulphur has been derived from sanskrit word ‘Sulvezi’ meaning ‘killer of copper’.
General Physical Properties of Group 16 Elements
(i) Electronic configuration Their valence shell electronic configuration is ns2, np4.
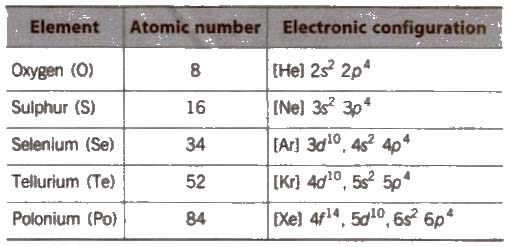
(ii) Metallic and non-metallic character

(iii) Abundance O > S > Se > Te > Po
(iv) Density It increases down the group regularly,
(v) Melting point and boiling point Both show a regular increase down the group due to increase in molecular weight and van der Waals’ forces of attraction.
(vi) Oxidation state
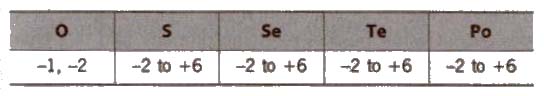
In OF2, the oxidation state of oxygen is +2.
(vii) Ionisation energy They possess a large amount of ionisation energy which decreases gradually from 0 to Po due to increase in size of atoms and increase in screening effect.
(viii) Electron affinity They have high electron affinity which decrease from O to Po. As the size of the atom increases. the extra added electron feels lesser attraction by nucleus and hence, electron affinity decreases.
(ix) Electronegativity It decreases down the group due to decrease in effective nuclear charge down the group.
(x) Catenation 16 group elements follow the order as shown below
S-S > Se-Se > O-O > Te-Te
(xi) Atomicity Oxygen is diatomic, sulphur and selenium are octaatomic with puckered ring structure.
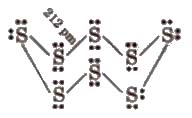
(xii) Allotropy
Oxygen – Dioxygen (O2) and ozone (O3)
Sulphur – Rhombic (01′ a) sulphur. Ss
Monoclinic (or β) sulphur, S8(most stable), plastic sulphur
(xiii) Atomic radii and ionic radiiThey increase regularly from O to Po.
Chemical Properties of 16 Group Elements
(i) Hydrides All these elements form stable hydrides of the type H2E. (Where. E = 0, S, Se, Te and Po).
2H2 + O2 ⇔ 2H2O
FeS + H2SO4 → H2S + FeSO4
H2O is a liquid due to hydrogen bonding. While others are colourless gases with unpleasant smell.
[Down the group acidic character increases from H2O to H2Se. All the hydrides except water possess reducing property and this character increases from H2 S to H2Te.
(ii) Halides The stability of the halides decreases in the order
F– > Cl– > Br– > 1–
Amongst hexahalides, hexafluorides are the only stable halides. AD hexafluorides are gaseous in nature. SF6 is exceptionally stable for steric reasons.
SF4 is a gas, SeF4 is a liquid and TeF4is a solid. These fluoride have sp3 d-hybridisation and see-saw geometry. They behave Lewis acid as well as Lewis base e.g.,
SF4 + BF3 → SF4 → BF3
SeF4 + 2F– → [SeF6]2-
The well known mono halides are dimeric in nature. Example are S2F2, S2C12, S2Br2, Se2C12 and Se2Br2. These dimeric halides undergo disproportionation as given below
2 SeCI2 → SeCI4 + Se
(ill) Oxides They form AO2 and AO3type oxides. Their acidic nature follow the order
SO2 > SeO2 > TeO2 > PoO2 and SO3> SeO3 > TeO3
Ozone is considered as oxides of oxygen.
SO2 is a gas having sps -hybridisation and V-shape.
SO3 is a gas which is sp2-hybridised and planar in nature.
SeO2 is a volatile solid consists of non-planar infinite chains.
SeO3 has tetrameric cyclic structure in solid state. SO2 and SO3 are the anhydrides of sulphurous (H2SO3) and sulphuric acid (H2SO4) respectively.
SO3 is a gas which is sp2-hybridised and planar in nature.
SeO2 is a volatile solid consists of non-planar infinite chains.
SeO3 has tetrameric cyclic structure in solid state. SO2 and SO3 are the anhydrides of sulphurous (H2SO3) and sulphuric acid (H2SO4) respectively.
Note In photocopying (xerox) machines Se acts as photoconductor.
Oxygen and its Compounds
1. Dioxygen
Priestley and Scheele prepared oxygen by heating suitable oxygen compounds.
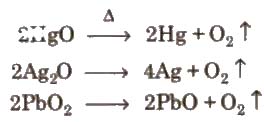
Preparation By action of heat on oxygen rich compounds
(i) From oxides
(ii) From peroxides and other oxides
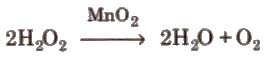
(iii) From certain compounds
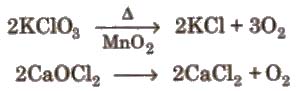
Physical properties It is colourless, odourless, tasteless, slightly heavier than air and sparingly soluble in water.
Chemical properties On heating it combines directly with metals and non-metals, e.g.,
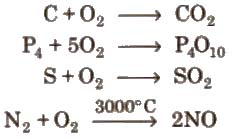
2Mg + O2 → 2MgO
4Na + O2 → 2 Na2O → Na2O2
Combination with O2 is accelerated by using catalyst. Platinum is particularly an active catalyst.
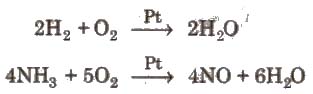
Uses It is used in welding and cutting oxy-hydrogen or oxy-acetylene torch and in iron and steel industry to increase the content of blast in the Bessemer and open hearth process. It is also used for life support systems e.g., in hospitals, for divers, miners and mountaineers.
Tests
1. With NO it gives reddish brown fumes of NO2.
2. It is adsorbed by alkaline pyrogallol.
2. Ozone (O3)
Preparation By passing silent electric discharge through cold, dry oxygen in ozoniser. (Lab method) –
3O2 ⇔ 2O3; + 284.3 kJ
Physical properties It is pale blue gas with characteristic strong smell. It is slightly soluble in water.
Chemical reactions
1.Decomposition
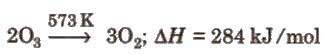
2. Oxidising action
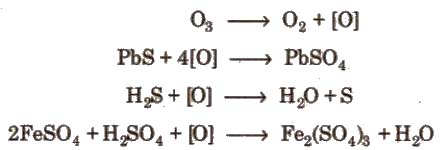
3. It acts as a powerful oxidising agent. It liberates iodine from neutral KI solution and the liberated L,turns starch paper blue.
2KI + H2 + O3 → 2KOH + I2 + O2
I2 + Starch → Blue colour
Uses It is used
1. as a germicide and disinfectant for sterilizing water.
2. ail a bleaching agent for oils, ivory wax and delicate fibres.
3. for detecting ‘the position of double bond in unsaturated compounds.
4. in destroying odours coming from cold storage room, slaughter houses and kitchen of hotels.
2. ail a bleaching agent for oils, ivory wax and delicate fibres.
3. for detecting ‘the position of double bond in unsaturated compounds.
4. in destroying odours coming from cold storage room, slaughter houses and kitchen of hotels.
compounds of Sulphur
1.Sulphur Dioxide (SO2)
Method of preparation
(i) By heating sulphur in air
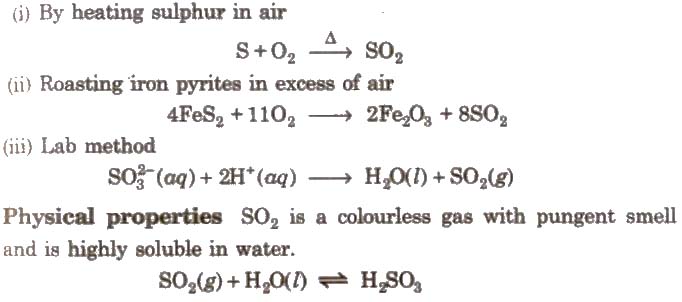
Chemical reactions It turns lime water milky due to the formation of calcium bisulphite. However, in excess of SO2 milkiness disappears due to the formation of calcium bisulphite.
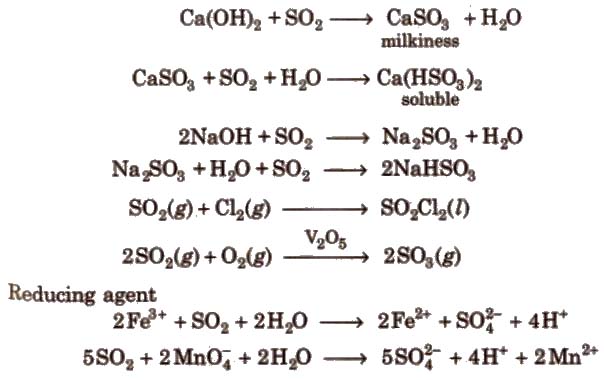
when H2S gas is passed through a saturated solution of SO2 till its smell disappears, it turns in a milky solution the Wacken roder’s liquid. When H2S is passed through H2SO4the reaction is called Wacken roder’s reaction.
Oxoacids of Sulphur
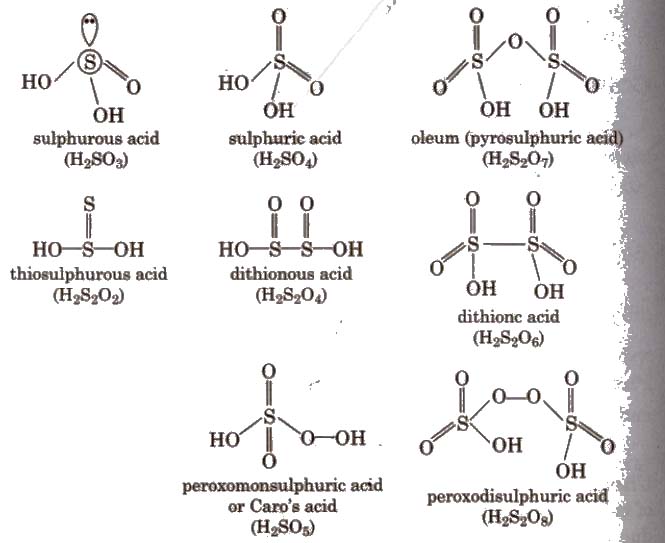
2. Sulphuric Acid (H2SO4)
Sulphuric acid is one of the most important industrial chemicals world wide. It is called the king of chemicals. It is manufactured by lead chamber process or contact process. Contact process involves three steps:
(i) Burning of sulphur or sulphur ores ill air to generate SO2.
(ii) Conversion of 2 to 2 by the reaction with oxygen in the presence of a catalyst (V2O5).
(iii) Absorption of SO3 in H2SO4 to give oleum (H2S2O7) which upon
hydrolysis gives H2SO4.
hydrolysis gives H2SO4.
Properties
1. Sulphuric acid is a colourless, dense, oily liquid.
MX + H2SO4 → 2HX + M2SO4
2. Concentrated sulphuric acid is a strong dehydrating agent.
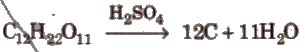
The burning sensation of concentrated H2SO4 on skin.
3. Hot concentrated sulphuric acid is a moderately strong oxidising agent. In this respect, it is intermediate between phosphoric acid and nitric acid.
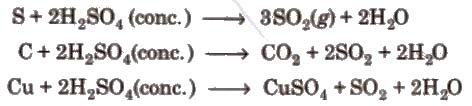
Uses It is used in petroleum refining, in pigments paints and in detergents manufacturing.
3. Hypo
It is chemically sodium thiosulphate pentahydrate, Na2S2O3 * 5H2O.
Preparation 1. It is prepared by boiling sodium sulphite solution with flowers of sulphur and stirring till the alkaline reaction has disappeared.
Na2SO3 + S → Na2S2O3
2. It is also prepared by spring’s reaction.
Na2S + Na2SO3 + I2 → Na2S2O3+2NaI
Properties 1. It is a colourless, crystalline and efflorescent substance.
2. It gives white ppt with a dilute solution of AgNO3. Which quickly changes into black due to the formation of Ag2S.
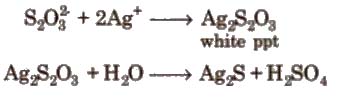
Uses
1. Due to its property of dissolving silver halide, it is used in photography for fixing under the name hypo.
2 Na2S2O3 + AgBr → Na3 [ Ag(S2O3)2] + NaBr
2. During bleaching, it is used as antichlor,
Na2S2O3 + CI2 + H2O → Na2SO4 + S + 2HCI
3. It is used to remove iodine stain, for volumetric estimation of iodine and in medicines.
Group 17
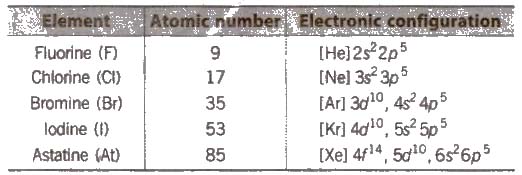
The 17 group of Periodic Table contains five elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (As) combinedly known as halogens (salt forming elements). Astatine is artificially prepared radioactive element.
General Physical Properties of Group 17 Elements
(i) Electronic configuration Their valence shell electronic configuration is ns2, np5
(ii) Physical state Intermolecular forces in halogens are weak and increase down the group. Thus, F2and Cl2 are gases, Br2 is volatile liquid and I2 is solid.
(iii) Atomicity All are diatomic in nature.
(iv) Abundance Being very reactive in nature, they are not found free in nature. Their presence in earth’s crust follows the order.
F2 > Cl2 > Br2 > I2
(v) Colour They absorb light in the visible range forming excited states and are thus, coloured in nature.

(vi) Metallic character All the elements are non-metals and metallic character increases down the group. Thus, 1 forms 1+.
(vii) Oxidation state

(viii) Bond energy and bond length The bond length increases from fluorine to iodine and in the same order bond energy decreases However, the bond dissociation energy of F2 is lesser due to its smaller size. The order of bond energy is
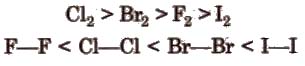
(he) Density It increases down the group in a regular fashion and follows the order F > Cl > Br > 1.
(x) Ionisation enthalpy The ionisation enthalpy of halogens is very high and decreases down the group. The iodine also forms I+ and I3+ and forms compounds like leI, lCN, IPO4. In molten state, the compounds conduct electricity showing ionic character.
(xi) Electron affinity The halogens have the high values for electron affinity. The order of electron affinity is
C12 > F2 > Br2 > I2
Due to small size of fluorine (hence, high electron density), the extra electron to be added feels more electron-electron repulsion. Therefore. fluorine has less value for electron affinity than chlorine.
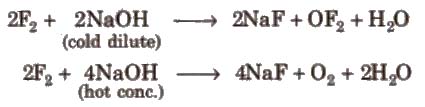
(xii) Reduction potentials and oxidising nature E°red of halogens are positive and decrease from F to I. Therefore, halogens act as strong oxidising agents and their oxidising power decreases from fluorine to iodine. Fluorine is the strongest oxidising agent and is most reactive. That’s why it is prepared by the electrolysis of a mixture of KHF2 and anhydrous HF using Monel metal as a catalyst.
(xii) Solubility Halogens are soluble in water which follows the order
F2 > C12 > Br2 > I2
The solubility of iodine in water is enhanced in the presence of KI.
KI + I2 ⇔ KI3 ⇔ K+ + I–2
I2 forms blue colour complex with starch.
Chemical Properties of Group 17 Elements
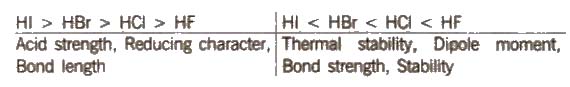
(i) Hydrides HF is a low boiling liquid due to intermolecular hydrogen bonding, while HCI, HBr, HI are gases. The boiling point follows the trend
HF > Hi > HBr > HCl
Some other properties show the following trend :
(ii) Oxides Fluorine forms two oxides, OF2 and O2F2, but only OF2is thermally stable at 2.98K O2F2oxidises plutonium to PuF6 and the reaction is used for removing plutonium as PuF6 from spent nuclear fuel.
Chlorine forms a number of oxides such as, CI2O, CI2O3, Cl2O5 , Cl2O7 , CIO2 and CIO2 is used as a bleaching agent for paper pulp, textiles and in water treatment.
Br2O BrO2 BrO3 are the least stable bromine oxides and exist only at low temperatures. They are very powerful oxidising agents.
The iodine oxides, i:e., I2O4, I2O5,I2O7 are insoluble solids and decompose on heating. I2O5 is a very good oxidising agent and is used in the estimation of carbon monoxide.
(iii) Reaction with alkali
Other halogens form hypohalite with dilute NaOH and halate with cone. NaOH4.
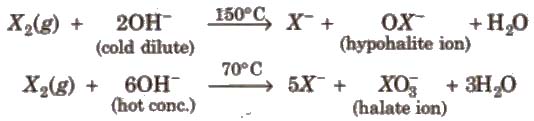
(iv) Oxoacids of halogens Higher oxoacids of fluorine such as HFO2, HFO3 do not exist because fluorine. is most electronegative
and has absence of d-orbitals.
and has absence of d-orbitals.
+3 oxidation state of bromine and iodine are unstable due to inert pair effect. therefore, HBrO2 and HIO2. do not exist.
Acidic character of oxoacids decreases as the electronegativity of halogen atom decreases. Thus, the order of acidic strength.

For the oxoacids of same halogens. acidic strength and thermal stability increase as the number of O atoms increases
Interhalogen Compounds
When two different halogens react with each other, interhalogen compounds are formed. These compounds are covalent and diamagnetic in nature. They are volatile solids or liquids except elf which is a gas at 298 K. Interhalogen compounds are more reactive than halogens (except fluorine).
The XY3 type compounds have bent ‘T’ shape, XY5 type compounds have square pyramidal shape and IF7has pentagonal bipyramidal structure.
BrF3 has “T” shaped structure due to 3 bp and 2 lp.
ICI is more reactive than I2 due to weak bond. ClF3 and BrF3 are used for the production of UF6 in the enrichment of 235 U.
U(s) + 3CIF3(I) → UF6(g) + 3CIF(g)
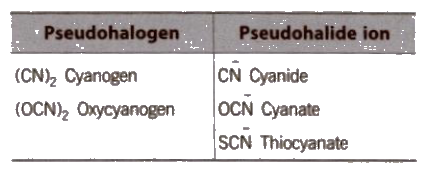
Pseudohalogens and Pseudohalides
The substances behaving like halogens are known as pseudohalides. Some examples are
Chlorine and its Compounds
Occurrence
Common salt, NaCI is most important. Chlorine is also present in sea water and as rock salt.
Preparation of Chlorine
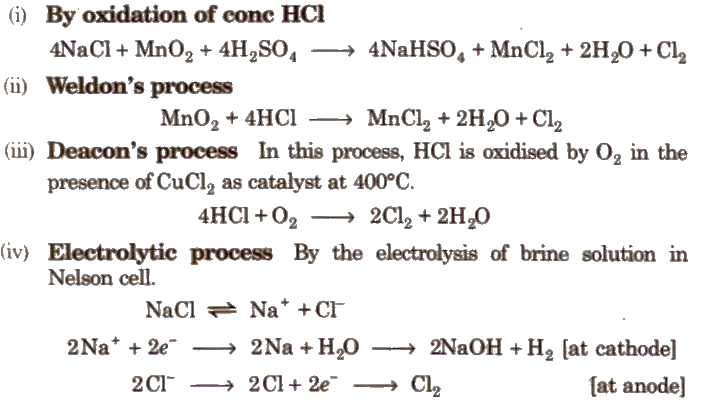
Properties
It is yellowish green gas, collected by upward displacement of air poisonous in nature, soluble in water. It’s aqueous solution is known as chlorine water.
Chemical Reactions
(i) Action of water
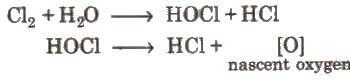
Coloured matter + [0] → colourless matter.
The bleaching action of chlorine is due to oxidation and is permanent.
(x) Chromyl chloride test When a mixture of chloride and solid K2Cr2O7 is heated with concentrated H2SO4 in a dry test tube, deep red vapours of chromyl chloride are evolved.
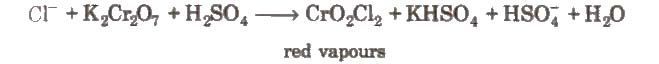
When these vapours are passed through NaOH solution, the solution becomes yellow due to the formation of sodium chromate
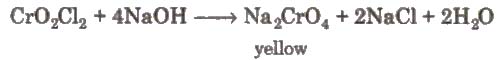
The yellow solution is neutralised with acetic acid and on addition of lead acetate gives a yellow precipitate of lead chromate.
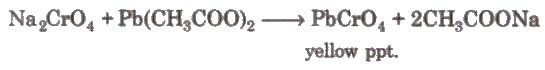
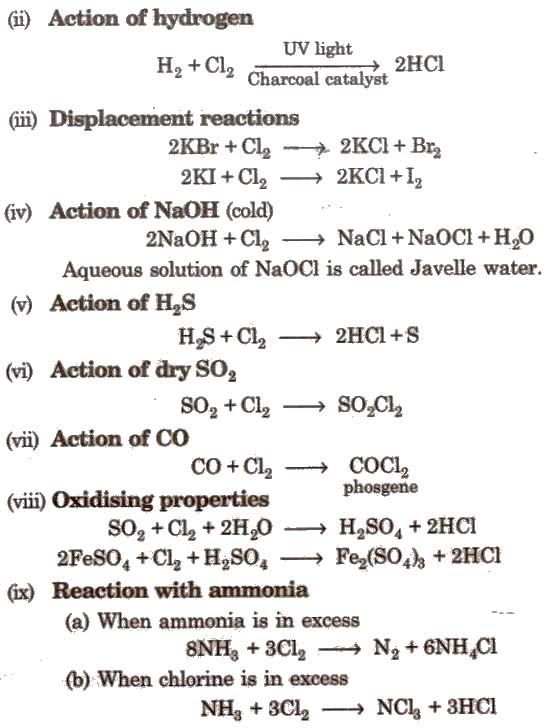
Uses
It is used as a bleaching agent, disinfectant and in the manufacture of CHCl3,CCl4, DDT, anti-knocking compounds and bleaching powder.
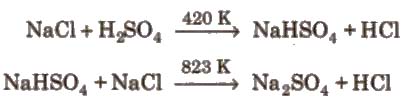
Hydrochloric Acid (Hel)
Preparation
Properties
It is a colourless and pungent smelling gas. It is extremely soluble in water and ionises as below
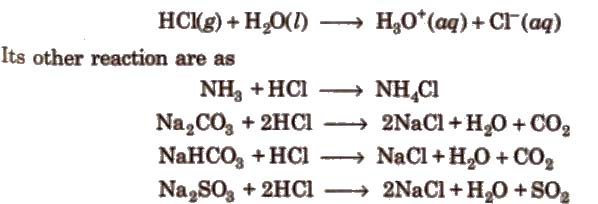
Noble metals like gold, platinum can dissolve in aqua-regia [three part cone HCl and one part of cone HNO3].
Uses
It is used in the manufacture of chlorides. chlorine, in textile and dyeing industries, in medicine and in extraction of glue from animal tissues and bones.
Iodine (I2)
It’s major SOurce is deep sea weeds of laminaria variety. Their ashes which is called kelp contain 0.5% iodine as iodides.
Another source of 12 is caliche or crude chile saltpetre (NaNO3) which contains 0.2%, NaIO3
Iodine is purified by sublimation.
It shows no reaction with water. Tincture of iodine is a mixture of I2and Kl dissolved in rectified spirit.
18 Group
The 18 group of the Periodic Table consists of colourless, odourless gases at room temperature, isolated by William Ramsay in 1898 from air
General/Physical Characteristics of Group 18 Elements
(i) Electronic configuration Their valence shell electronic configuration is ns2, np2 except He.
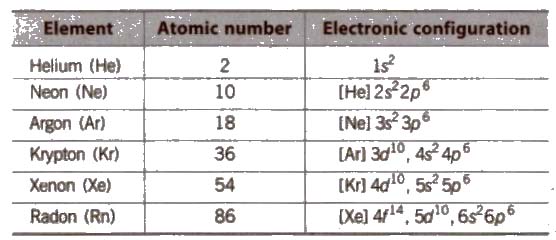
(ii) Physical state They are all gases under ordinary conditions of temperature and pressure.
(iii) Abundance In 1.O% air, the abundance follows the order
Ax > Ne > He > Kr > Xe
(iv) Atomicity The Cp / Cv = 1.67 shows their monoatomic nature.
However under high energy conditions, several molecular ions such as He+2, HeH+, HeH2+and Ar+2 are formed in discharge tubes. They only survive momentarily and are detected spectroscopically.
(v) Melting and boiling points Due to the increase in magnitude of van der Waals’ forces, the melting point and boiling point increases from He to Rn.
(vi) Atomic radii The atomic radii increases from He to Rn. It corresponds to the van der Waals’ radii. So it has greatest atomic size in respective period.
(vii) Density The density of noble gases increases down the group.
(viii) Heat of vaporisation They have very low values of heal of vaporisation due to weak van der Waals’ forces of attraction. The value increases down the group.
(ix) Solubility in water They are slightly soluble in water and solubility increases from He to Rn.
(x) Liqnefication It is extremely difficult to liquify inert gases due to weak van der Waals’ forces of attraction among their molecules. Hence, they posses low value of critical temperature also.
(xi) Ionisation energy All noble gases possess very stable (ns2 and ns2 np6) electronic configuration. Therefore. ionisation energy of noble gases is very high and decreases down the group.
(xii) Electron affinity Due to the presence of stable electronic configuration, they have no tendency to accept additional electron. Therefore, electron affinity is almost zero.
Chemical Properties of Group 18 Elements
The noble gases are inert in nature because of their completely filled subshells. In 1962, the first compound of noble gases was prepared. It is hexafluoroplatinate (prepared by Bartlett).
Xe + PtF6 → Xe[PtF6]
Now, many compounds of Xe and Kr are known with fluorine and oxygen.
Preparation of Compounds of Xenon
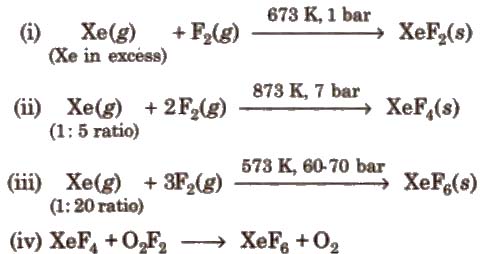
Chemical Reactions of Xenon Compounds


