Class 11 Chemistry Chapter Some Basic Concepts of Chemistry Notes

It is the branch of science which deals with the composition, structure and properties of matter.
Branches of Chemistry
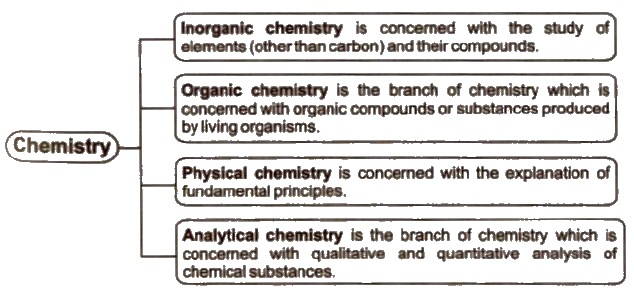
In addition to these biochemistry, war chemistry, nuclear chemistry forensic chemistry, earth chemistry etc., are other branches of chemistry.
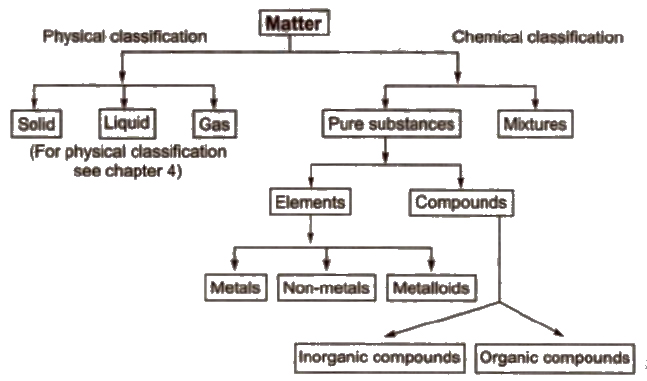
Matter
Anything which occupies some space and have some mass is called matter. It 15 made up of small particles which have space between them. The matter particles attract each other and are in a state of continuous monon.
Elements
It is the simplest form of pure substance, which can neither be decomposed into nor built from simpler substances by ordinary physical and chemical methods. It contains only one kind of atoms. The number of elements known till date is 118.
[Hydrogen IS the most abundant element in the universe.
OXYgen (46.6%), a non-metal, is the most abundant element in the earth crust.
AI is the most abundant metal in the earth crust.
An element can be a metal, a non-metal or a metalloid.]
Symbols
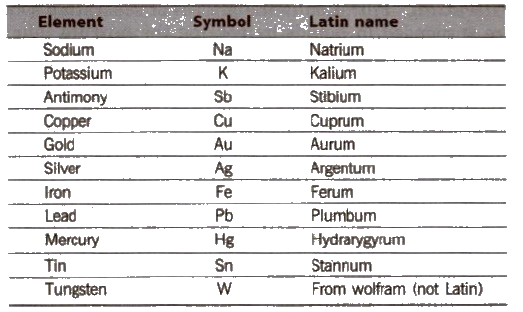
A symbol is an abbreviation or shortened form for the full name of an element. The present system of symbols was introduced by Berzelius.
Symbol and Latin Names for Some Elements
Compounds
It is also the form of matter which can be formed by combining two or more elements in a definite ratio by mass. It can be decomposed into its constituent elements by suitable chemical methods, e.g., water (H2O) is made of hydrogen and oxygen in the ratio 1 : 8 by mass.
Compounds can be of two types :
(i) Inorganic compoundsPreviously, it was believed that these compounds are derived from non-living sources, like rocks and minerals. But these are infact the compounds of all the elements except hydrides of carbon (hydrocarbons) and their derivatives.
(ii) Organic compounds According to earlier scientists, these compounds are derived from living sources like plants and animals, or these remain buried under the earth (e.g., petroleum). According to modern concept, these are the hydrides of carbon and their derivatives.
Mixtures
These are made up of two or more pure substances. They can possess variable composition and can be separated into their components by some physical methods.
Mixtures may be homogeneous (when composition is uniform throughout) or heterogeneous (when composition is not uniform throughout).
Common methods for the separation of mixtures are
(a) Filtration Filtration is the process of separating solids that are suspended in liquids by pouring the mixture into a filter funnel. As the liquid passes through the filter, the solid particles are held on the filter.
(b) Distillation Distillation is the process of heating a liquid to form vapours and then cooling the vapours to get back the liquid. This is a method by which a mixture containing volatile substances can be separated into Its components.
(c) Sublimation This is the process of conversion of a solid directly into vapours on heating. Substances showing this property are called sublimate, e.g., iodine, naphthalene, camphor. This method is used to separate a sublimate from non-sublimate substances.
(d) Crystallisation It is a process of separating solids having different solubilities in a particular solvent.
(e) Magnetic separation Tills process is based upon tbe fact that a magnet attracts magnetic components of a mixture of magnetic and non-magnetic substances. The non-magnetic substance remains unaffected. Thus. it can be used to separate magnetic components from non-magnetic components.
(f) Atmolysis Tills method is based upon rates of diffusion of gases and used for their separation from a gaseous mixture.
Atoms and Molecules
Atom is the smallest particle of an element which can take part in a chemical reaction. It mayor may not be capable of independent existence.
Molecule is the simplest particle of matter that has independent existence. It may be homoatomic e.g., H2, CI2, N2 (diatomic), O3(triatomic) or heteroatomic, e.g., HCI, NH3, CH3 etc.
Physical Quantities and Their Measurements
Units
To express the measurement of any physical quantity two things are considered:
(i) Its unit,
(ii) The numerical value.
Magnitude of a physical quantity = numerical value * unit
Units are of two types:
(i) Basic units
(ii) Derived units
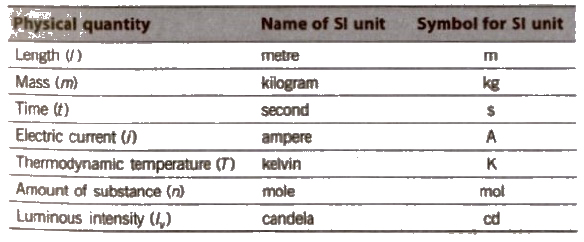
(i) The basic or fundamental units are those of length (m), ass (kg), time (s), electric current (A), thermodynamic temperature (K), amount of substance (mol) and luminous intensity (cd).
(ii) Derived units are basically derived from the fundamental units, e.g., unit of density is derived from units of mass and volume.
The systems used for describing measurements of various physical quantities are
(a) CGS system It is based on centimetre, gram and second as the units of length, mass and time respectively.
(b) FPS system A British system which used foot(ft). pound (lb) and second (s) as the fundamental units of length, mass and time.
(c) MRS system Uses metre (m), kilogram (kg) and second (s) respectively for length, mass and time; ampere (A) was added later on for electric current.
(d) SI system (1960)International system of units and contains following seven basic and two supplementary units:
Base Physical Quantities and their Corresponding Basic Units
SUpplementary units It includes plane angle in radian and solid angle in steradian.
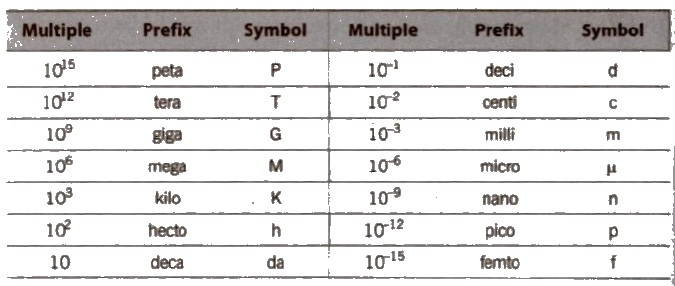
Prefixes
The SI units of some physical quantities are either too small or too large. To change the order of magnitude. these are expressed by using prefixes before the name of base units. The various prefixes are listed as
Dimensional Analysis
Often while calculating, there is a need to convert units from one system to other. The method used to accomplish this is called factor label method or unit factor method or dimensional analysis.
In this,
Information sought = Information given * Conversion Factor
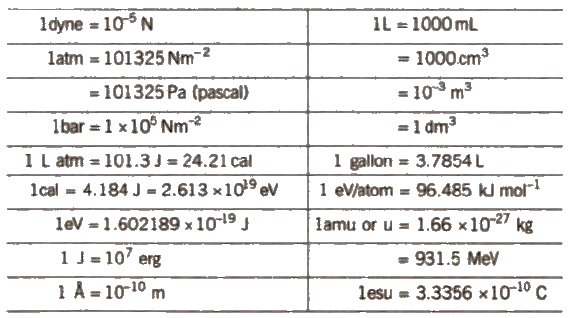
Important Conversion Factor
Scientific Notation
In such notation, all measurements (however large or small) are expressed as a number between 1.000 and 9.999 multiplied or divided by 10. In general as
N * 10
Here, N is called digit term (1.000-9.999) and n is known as exponent. e.g., 138.42 cm can be written as 1.3842 * 102 and 0.0002 can be written as 2.0 * 10-4.
precision and Accuracy
Precision refers the closeness of the set of values obtained from identical measurements of a quantity. Precision is simply a measure of
reproducibility of an experiment.
reproducibility of an experiment.
Precision = individual value – arithmetic mean value
Accuracy is a measure of the difference between the experimental value or the mean value of a set of measurements and the true value.
Accuracy = mean value – true value
In physical measurements, accurate results are generally precise but precise results need not be accurate. In other words good precision does not assure good accuracy.
Significant Figures
Significant figures are the meaningful digits in a measured or calculated quantity. It includes all those digits that are known with certainty plus one more which is uncertain or estimated.
Greater the number of significant figures in a measurement, smaller the uncertainty.
Rules for determining the number of significant figures are:
1. An digits are significant except zeros in the beginning of a number.
2. Zeros to the right of the decimal point are significant. e.g., 0.132, 0.0132 and 15.0, all have three significant figures.
3. Exact numbers have infinite significant figures.
Calculations Involving Significant Figures
1. In addition or subtraction, the final result should be reported to the same number of decimal places as that of the term with the least number of decimal places, e.g.,
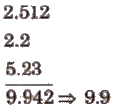
(Reported sum should have only one decimal point.)
2. In multiplication and division, the result is reported to the same number of significant figures as least precise term or the term
with least number of significant figures, e.g.,
with least number of significant figures, e.g.,
Rounding Off the Numerical Results
When a number is rounded off, the number of significant figures is reduced. the last digit retained is increased by 1 only if the following digit is ≥ 5 and is left as such if the following digit is ≤ 4, e.g.,
12.696 can be written as 12.7
18.35 can be written as 18.4
13.93 can be written as 13.9
Laws of Chemical Combinations
The combination of elements to form compounds is governed by the following six basic laws:
1. Law of conservation of mass(Lavoisier, 1774)
This law states that during any physical or chemical change, the total mass of the products is equal to the total mass of reactants. It does not hold good for nuclear reactions.
2. Law of definite proportions(Proust, 1799)
According to this law, a chemical compound obtained by different sources always contains same percentage of each constituent element.
3. Law of multiple proportions (Dalton, 1803)
According to this law. if two elements can combine to form more than one compound. the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers, e.g., in NH3and N2H4, fixed mass of nitrogen requires hydrogen in the ratio 3 : 2.
4. Law of reciprocal proportions(Richter, 1792)
According to this law, when two elements (say A and 13) combine separately with the same weight of a third element (say C), the ratio in which they do so is the same or simple multiple of the ratio in which they (A and H) combine with each other.
Law of definite proportions, law of multiple proportions and law of reciprocal proportions do not hold good when same compound is obtained by using different isotopes of the same element. e.g H2O and D2O
5. Gay Lussac’s law of gaseous volumes
It states that under similar conditions of temperature and pressure. whenever gases react together. the volumes of the reacting gases as well as products (if gases) bear a simple whole number ratio.
6. Avogadro’s hypothesis
It states that equal volumes of all gases under the same conditions of temperature and pressure contain the same number of molecules.
Dalton’s Atomic Theory (1803)
This theory was based on laws of chemical combinations. It’s basic postulates are
1. All substances are made up of tiny. indivisible particles, called atoms.
2. In each element, the atoms are all alike and have the same mass. The atoms of different elements differ in mass.
3. Atoms can neither be created nor destroyed during any physical or chemical change.
4. Compounds or molecules result from combination of atoms in some simple numerical ratio.
Mole Concept
Term mole was suggested by Ostwald (Latin word mole = heap)
A mole is defined as the amount of substance which contains same number of elementary particles (atoms, molecules or ions) as the number of atoms present in 12 g of carbon (C-12).
1 mol = 6.023 * 1023 atoms = one gram-atom = gram atomic mass
1 mol = 6.023 * 1023 molecules = gram molecular mass
In gaseous state at STP (T = 273 K, p = 1 atm)
Gram molecular mass = 1 mol
= 22.4 L = 6.022 * 1023 molecules
Standard number 6.023x 1023 is called Avogadro number in honour of Avogadro (he did not give this number) and is denoted by NA.
The volume occupied by one mole molecules of a gaseous substance is called molar volume or gram molecular volume.
Number of moles = amount of substance (in gram) / molar mass
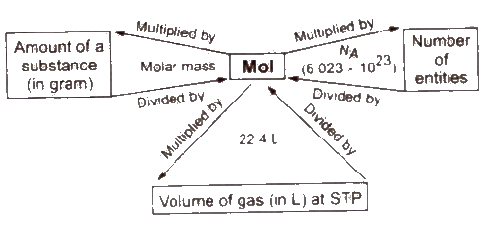
Number of molecule = number of moles * NA
Number of molecules in Ig compound = NA / g-molar mass
Number of molecules in 1 cm3 (1 mL) of an ideal gas at STP is called Loschmidt number (2.69x 1019).
[One amu or u (unified mass) is equal to exactly the 1 / 12th of the mass of 12C atom, i.e., 1 amu or u = 1 / 12 * mass of one carbon (C12) atom
1 amu = 1 / NA
= 1 Dalton = 1.66x 10-24 g
One mole of electrons weighs 0.55 mg (5.5x 10-4 g).
Atomic Mass
It is the average relative atomic mass of an atom. It indicates that how many times an atom of that element is heavier as compared with 1 / 12 of the mass of an atom of carbon-12.
Average atomic mass = average mass of an atom / 1 / 12 * mass of an atom of C12
The word average has been used in the above definition and is very significant because elements occur in nature as mixture of several isotopes. So. atomic mass can be computed as
Average atomic mass
= RA(1) * at. mass(1) + RA(2) * at. mass (2) / RA(l) + RA(2)
Here, RA is relative abundance of different isotopes.
In case of volatile chlorides. the atomic weight is calculated as
At. wt. = Eq. wt. x valency
and valency = 2 * vapour density of chloride / eq. wt. of metal + 35.5
According to Dulong and Petit’s rule,
Atomic weight * specific heat = 6.4
Gram Atomic Mass (GAM)
Atomic mass of an element expressed in gram is called its gram atomic mass or gram-atom or mole-atom.
Molecular Mass
It is the mass of a molecule, i.e., number of times a molecule is heavier than 1 / 12 th mass of C-12 atom. Molecular mass of a substance is an additive property and can be calculated by taking algebraic sum of atomic masses of all the atoms of different elements present in one molecule.
Molecular Mass = average relative mass of one molecule / 1 / 12 th * mass of C-12 atom
[Gram molecular mass or molar mass is molecular mass of a substance expressed in gram.
Molecular mass = 2 * V D ]
Equivalent Mass
It is the mass of an element or a compound which would combine with or displace (by weight) 1 part of hydrogen or 8 parts of oxygen or 35.5 parts of chlorine
Eq. wt. of metal = wt. of metal / wt. of H2 displaced * 1.008
= wt. of metal / volume of H2 (in mL) displaced at STP * 11200
Eq. wt. of metal = wt. of metal / wt. of oxygen combined * 8
= wt. of metal / wt. of chlorine combined * 35.5
In general,
Wt. of substance A / Wt. of substance B = Eq. wt. of A / Eq. wt. of B
or for a compound (I) being converted into another compound (II) of same metal
Wt. of compound I / Wt. of compound II
= eq. wt. of metal + eq. wt. of anion of compound I / eq. wt. of metal + eq. wt. of anion of compound II
Eq. mass 0f a salt = formula mass / total positive or negative charge
Equivalent mass = atomic mass or Molecular mass / n factor
n factor for various compounds can be obtained as
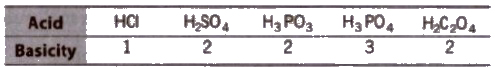
(i) n factor for acids i.e., basicity
(Number of ionisable H+ per molecule is the basicity of acid.)
(ii) n factor for bases, i.e., acidity.
(Number of ionisable OH– per molecule is the acidity of a base.)

(iii) In case of ions, 11 factor is equal to charge of that ion.
(iv) In redox titrations, 11 factor is equal to change in oxidation number.
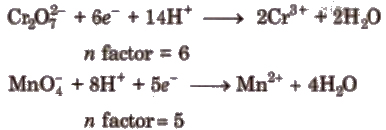
Equivalent mass of organic acid (RCOOH) is calculated by the following formula
Eq. wt. of silver salt of acid (RCOOAg) / Eq. wt. of Ag(or 108) = Vt. of silver salt / Wt. of silver
Stoichiometry
The relative proportions in which the reactants react and the products are formed, is called stoichiometry (from the Greek word meaning ‘to measure an element’.)
Limiting reagent It is the reactant which is completely consumed during the reaction.
Excess reagent It is the reactant which is not completely consumed and remains unreacted during the reaction.
[In a irreversible chemical reaction, the extent of product can be computed on the basis of limiting reagent in the chemical reaction]
Percent Yield
The actual yield of a product in any reaction is usually less than the theoretical yield because of the occurrence of certain side reactions.
Percent yield = actual yield / theoretical yield * 100
Empirical and Molecular Formulae
Empirical formula is the simplest formula of a compound giving simplest whole number ratio of atoms present in one molecule, e.g., CH is empirical formula of benzene (C6H6).
Molecular formula is the actual formula of a compound showing the total number of atoms of constituent elements, e.g., C6H6 is molecular formula of benzene.
Molecular formula = (Empirical formula)n
where, n is simple whole number having values 1, 2, 3, … , etc., and can be calculated as
n = molecular formula mass / empirical formula mass

